Geochemical characteristics of titanite and U-Pb age of garnet from mineral mines of the Southern Urals
- 1 — Postgraduate Student Empress Catherine II Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 2 — Ph.D., Dr.Sci. Director Institute of Precambrian Geology and Geochronology RAS ▪ Orcid
- 3 — Ph.D. Associate Professor Empress Catherine II Saint Petersburg Mining University ▪ Orcid ▪ Scopus
- 4 — Ph.D. Senior Researcher Institute of Precambrian Geology and Geochronology RAS ▪ Orcid
- 5 — Researcher Institute of Precambrian Geology and Geochronology RAS ▪ Orcid
Abstract
Mineralogical-geochemical (SIMS method) study of titanite and geochronological (ID-TIMS method) study of garnets from mineral mines of the Southern Urals has been carried out. The mineral associations containing titanite belong to four contrasting types: epidote-titanite-garnet (Akhmatovskaya mine); garnet-titanite-diopside (Akhmatovskaya mine); epidote-titanite-chlorite (Nikolaje-Maksimilianovskaya mine); chlorite-titanite-garnet (Praskovie-Evgenyevskaya mine). Titanite from mineral aggregates of the Akhmatovskaya mine is enriched with LREE and Th, Nikolaje-Maksimilianovskaya mine – with HREE, Hf and Ta, Praskovie-Evgenyevskaya mine – with V, Cr, and Sr. It was found that the distribution of trace and rare-earth elements in titanite is related both to the composition of paragenetic minerals (garnet and epidote, Akhmatovskaya and Nikolaje-Maksimilianovskaya mines) and to the influence of rock-forming minerals of the parent rock – gabbro (plagioclase and pyroxene, Praskovie-Evgenyevskaya mine). The age of garnets (504.1±4 Ma) determined by ID-TIMS method from silicate-carbonate rocks of the Perovskitovaya mine does not agree with the ideas about the formation of the latter as a result of contact metasomatosis synchronous with the introduction of gabbroids or granitoids of the Kusa-Kopan complex (1,390-1,350 Ma), but does not exclude the influence of superimposed contact metasomatosis associated with late endogenous processes.
Funding
The work was carried out within the framework of the State assignment of the IPGG RAS FMUW-2022-0005.
Introduction
Titanite, CaTi[SiO4](O) is a common accessory mineral in rocks of various genesis: igneous, metamorphic, metasomatic (including contact-metasomatic and hydrothermal). The crystallochemical position of Ca2+ can be isomorphously replaced by Na+, K+, Mn2+, Ba2+, Sr2+, Y3+, REE3+, U4+, Th4+, the position of Ti4+ – Mg2+, Fe2+, Fe3+, Al3+, Cr3+, V3+, Sc3+, Zr4+, Sn4+, Hf4+, Nb5+, Ta5+, and Si4+ – Ti4+ and P5+ [1-3]. The anionic position of O2– can isomorphically include F–, Cl– and the hydroxyl group ОН–. Titanite is a geochronometer mineral due to the presence of uranium and the relatively high closing temperature of the U-Pb isotope system – about 700 °C [4-6]. Titanite is also used to estimate temperature and pressure [7-9]. Due to its ability to accumulate trace and rare-earth elements (REE), the content of which can change dramatically under the action of superimposed processes, the results of REE analysis can make a significant contribution to solving the issues of petrogenesis [10-12].
The main feature of magmatic titanite is a significant accumulation of LREE relative to HREE [13]. During metamorphic and metasomatic processes, the ratio of LREE and HREE in titanite can vary greatly [14-16]. In some cases, during high-temperature hydrothermal action on titanite, HREE may dominate over LREE [17, 18]. In general, REE fractionation in titanite during its formation is determined by various factors: temperature, pressure, and mineral paragenesis [19]. Nevertheless, the distribution of trace and rare-earth elements in titanite from rocks of metasomatic origin has not been well studied to date.
In this work we studied the peculiarities of the chemical composition of titanite by major, trace and rare-earth elements from mineral aggregates formed among rocks with skarn and rodingite associations from the Akhmatovskaya, Nikolaje-Maksimilianovskaya and Praskovie-Evgenyevskaya mines. The mineral mines are located along the western boundary of the Middle Riphean Kusa-Kopan clinopyroxenite-gabbro-granite intrusive complex with Lower Riphean sedimentary rocks of the Satka Formation in the Southern Urals. The Akhmatovskaya mine is located 16 km north of the town of Zlatoust (Chelyabinsk Region). A series of small mine workings, the Nikolaje-Maksimilianovskaya mine, is located 3 km northeast of the Akhmatovskaya mine. The Praskovie-Evgenyevskaya mine is located 15 km southwest of the Akhmatovskaya mine in the territory of the Medvedevskoye titanomagnetite deposit open pit. The mines were the first to discover dozens of new mineral species [20]. The diverse mineral associations and paragenesis manifested in the mines have been widely characterized by various researchers, but data on the chemical composition of rock-forming minerals are either fragmentary or absent. For titanite, which was determined as the most common accessory mineral in rocks from the Akhmatovskaya [21], Nikolaje-Maksimilianovskaya [22], and Praskovie-Evgenyevskaya [23] mines, there are no data on the content of trace and rare-earth elements.
The problem of age estimation of rocks uncovered by mineral mine in the Southern Urals is also open, as well as the problem of dating metasomatic formations. Due to the absence of classical geochronometer minerals (e.g., zircon [24]) in the rocks, U-Pb dating of an unconventional geochronometer mineral – calcium garnet (rich in U) from silicate-carbonate rocks of the Perovskitovaya mine, which is located in the Satka Formation and is situated between the Akhmatovskaya and Praskovie-Evgenyevskaya mines, was carried out using the ID-TIMS method. These data complement the study of the composition of garnets from silicate-carbonate rocks [25].
Methods
For the mineralogical and geochemical study of titanite, the scientific and auxiliary fund of the Mining Museum of Empress Catherine IISaint Petersburg Mining University provided four pieces from the study collection: 851/7 – medium-crystalline aggregate of diopside with titanite and garnet (andradite-grossular series) on chlorite-pyroxene rock (Akhmatovskaya mine); 851/34 – large-medium crystalline aggregate with epidote-titanite-clinochlore association on chlorite schist (Nikolaje-Maksimilianovskaya mine); 851/42 and 851/44 – medium crystalline aggregates of chlorite and titanite with garnet (andradite-grossular series) on amphibole-pyroxene-plagioclase rock or gabbro (Praskovie-Evgenyevskaya mine). In addition, titanite from medium crystalline epidote-titanite-garnets druse developed on pyroxene-garnet-chlorite rock selected by the authors among the rocks of the Akhmatovskaya mine (Akhm-12) was studied.
Mineralogical study of the samples and description of the relationship between the mineral associations were carried out at the Department of Mineralogy, Crystallography and Petrography of Mining University. Photographs were taken at the Mining Museum using an OLYMPUS SZX16 stereomicroscope with a built-in DP74 camera. To estimate the content of major, trace and rare-earth elements, 19 titanite grains of comparable size (from 1 to 2 mm) from different mineral associations were selected: epidote-titanite-garnet (Akhmatovskaya mine, 3 grains); garnet-titanite-diopside (Akhmatovskaya mine, 3 grains); epidote-titanite-chlorite (Nikolaje-Maksimilianovskaya mine, 5 grains); chlorite-titanite-garnet (Praskovie-Evgenyevskaya mine, 4 grains); garnet-titanite-chlorite (Praskovie-Evgenyevskaya mine, 4 grains). Geochemical study of titanite was carried out after preliminary manufacture of standard preparations (washers) from epoxy resin with a diameter of 2.5 cm. The washers were ground and polished to the surface of approximately the middle of all titanite grains.
The major elements were determined at the Institute of Precambrian Geology and Geochronology of the RAS in the central and marginal parts of titanite grains by the SEM-EDS method on a JEOL JSM-6510 LA scanning electron microscope with a JED-2200 energy dispersive spectrometer. Based on these data, the crystallochemical coefficients of titanite were calculated using the anionic method for 5 oxygen atoms (O = 5) [26].
The content of trace and rare-earth elements in the same parts of titanite grains was determined by secondary ion mass spectrometry (SIMS). The measurement was carried out on the ion probe Cameca IMS-4f in the Yaroslavl branch of the Institute of Physics and Technology of the RAS. The size of the analysis area did not exceed 20 μm in diameter. The relative error of measurement did not exceed 10-15 %. The detection threshold was 0.005-0.010 ppm. When plotting the distribution spectra the REE content in titanite was normalized to the composition of chondrite CI [27].
To reveal the distribution patterns of rare and rare-earth elements in the considered titanite, the principal component analysis (PCA) method was applied [28-30]. This method is successfully applied to reveal the peculiarities of rare-element composition of titanite [19, 31, 32]. The results of the PCA method were obtained in the software package Statistica 10 for pre-normalized analytical data (by logarithmization) [33]. The calculations of the titanite formation temperature were made using a geothermometer of Zr in titanite [7].
Geochronological study of garnet from silicate-carbonate rocks of the Perovskitovaya mine was carried out by U-Pb method (ID-TIMS, IPGG RAS). Preliminary isolated garnet grains up to 2 mm in size were cleaned from aggregates of other minerals in ultrapure water in an ultrasonic bath, then washed from surface contaminants with 2N or 3.3N HCl solution at 80 °C for 15 min and ultrapure water at the same temperature. Decomposition of garnet was carried out in metal bombs with Teflon liners in a mixture of concentrated acids: HF and HNO3 (3:1) at 220 °C. The separation of Pb from garnet was carried out by ion-exchange chromatography using Bio-Rad AG 1-X8 resin according to the HBr-HCl method [34]. U extraction was performed on UTEVA resin by Eichrome [35]. A mixed 235U+208Pb indicator was used to determine the Pb and U content. Isotopic ratios of Pb and U were measured on a Triton TI multicollector mass spectrometer. Contamination in the experiment did not exceed 50 pg for Pb and 2 pg for U. Processing of the results of Pb and U isotopic analysis and age calculation were performed using the PbDat program and the Microsoft Excel program add-in Isoplot 3.70. The construction of discordia by five figurative points was performed taking into account the error of U-Pb ratios equal to 0.5 %.
Results
Samples characterization
The sample Akhm-12 in the Akhmatovskaya mine is a chlorite-pyroxene-garnet rock (pyroxene-garnet skarn), which fracture or cavity is made by a coarser-grained epidote-titanite-garnet aggregate (Fig.1, a). Crystals of green columnar epidote are single, their size does not exceed 2 mm. Titanite has white or pale beige color and prismatic or wedge-shaped form, forms characteristic flattened crystals (“envelopes”) from the first millimeters to the first centimeters in size. Garnet is represented by solid masses (aggregates) of cherry-red to dark red crystals with a combination of tetragon-trioctahedral and rhombododecahedral habitus. Garnet grains vary in size from the first millimeters to the first centimeters. On the surfaces of facets between titanite and garnet crystals, as well as epidote and garnet, induction hatchings are observed, which indicate the joint formation of these minerals.
Sample 851/7 was selected among the rocks cut by the Akhmatovskaya mine. It is predominantly composed of chlorite-pyroxene rock (pyroxene skarn), in which fracture or cavity an aggregate of diopside, titanite, and garnet is formed (Fig.1, b). Crystals of dark red isometric garnet are either single or form small clusters of grains barely exceeding 1 mm in size. Titanite has a white or pale beige color, prismatic or tablitic appearance, and an average size of 1.5 mm. Pale green diopside is formed by solid masses and hexagonal aggregates without pronounced crystallographic outlines, which size researches 5 mm – idiomorphic grains of titanite and garnet are developed along them. At the same time, the contacting faces of titanite and garnet have developed induction hatching, indicating the simultaneous growth of these two minerals.
Stuff 851/34, selected among the rocks of the Nikolaje-Maksimilianovskaya mine, mainly consists of vein epidote-titanite-chlorite aggregate with small fragments of the parent rock – chlorite schist (Fig.1, c). Epidote of pistachio-green color and prismatic appearance varies in size from 1 to 10 mm. Crystals of titanite of pale beige color with greenish tinge are characterized by wedge-shaped forms and tabular appearance – their size reaches 1.5 cm. Chlorite (probably, clinochlore) of dark green (close to swamp-green or black) color forms large scale aggregates of crystals of hexagonal-plate or tablite form. Its size varies from the first millimeters and reaches 3-4 cm. In the zones of contact between the faces of epidote and titanite, titanite and chlorite, epidote and chlorite, induction shading is formed, which is an indicator of the joint growth of these minerals.
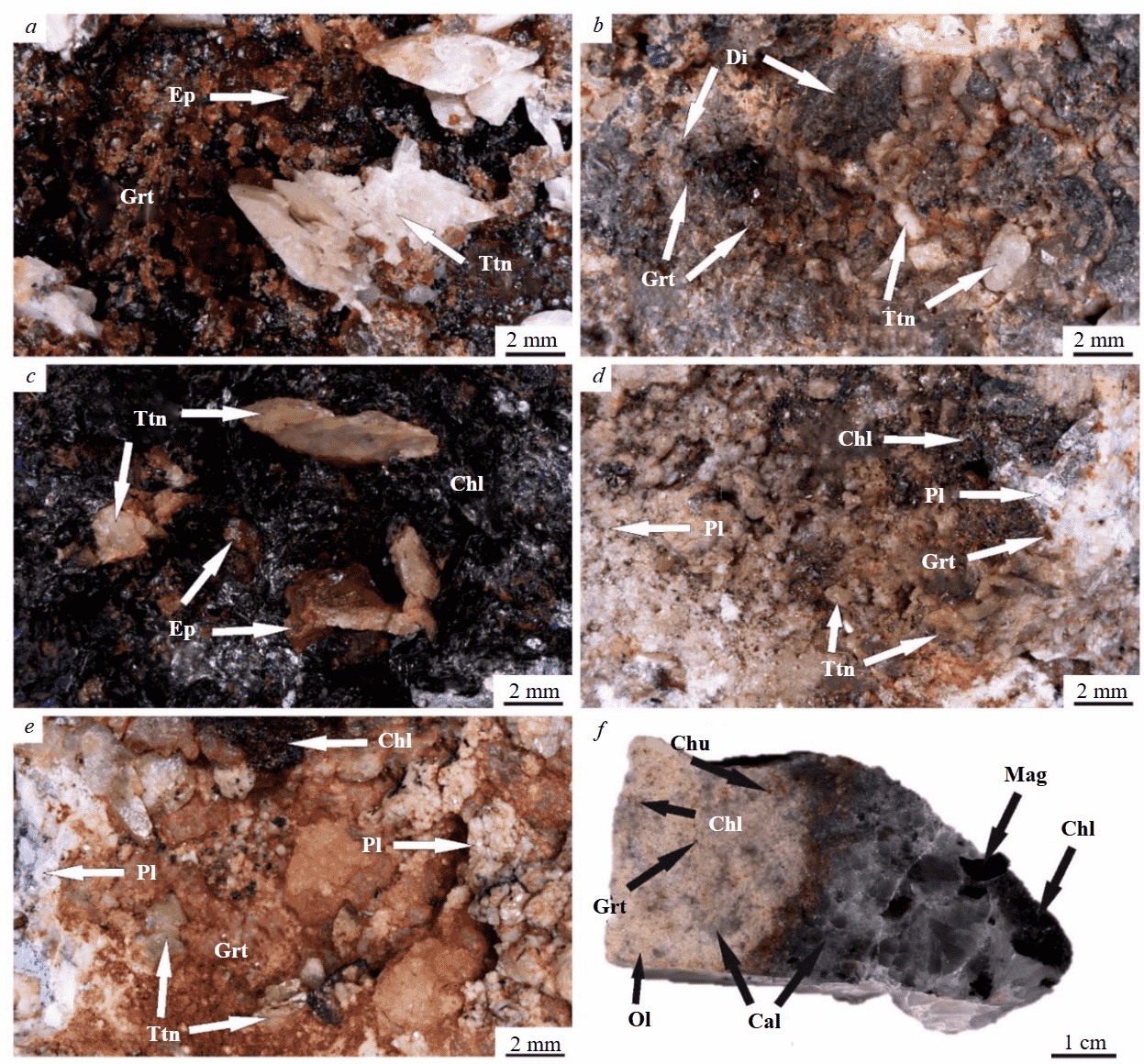
Fig.1. Titanite of different mineral associations: a – epidote-titanite-garnet (sam. Akhm-12); b – garnet-titanite-diopside (sam. 851/7); c – epidote-titanite-clinochlore (sam. 851/34); d, e – chlorite-titanite-garnet: d – garnet in subordinate quantity (sam. 851/42), e – garnet prevails (sam. 851/44); f – contact of silicate-carbonate rock and chlorite-calcite vein (sam. Prv-9)
Chl – clinochlore; Di – diopside; Ep – epidote; Grt – andradite-grossular garnet; Pl – plagioclase (albite – oligoclase – andesine); Ttn – titanite
Samples 851/42 and 851/44 were selected in the rocks cut by the Praskovie-Evgenyevskaya mine and are gabbro, where crystalline aggregates of chlorite and titanite with garnet of andradite-grossular series are formed on the walls of fractures or cavities. Stuffs differ from each other by the amount of garnet (Fig.1, d, e). Chlorite forms finely scaly aggregates of swamp-green (up to black) color up to 3 mm in size (the size of some individuals reaches fractions of a millimeter). Titanite has a light green color with a yellowish tinge and a flattened short-prismatic appearance – its size reaches 5 mm (average 2 mm). Garnet from honey to light red color forms solid granular masses without obvious crystallographic forms, the sizes of separate individuals are equal to fractions of a millimeter. The marked minerals grow on tablite-prismatic grains of white or beige plagioclase (albite, oligoclase or andesine). In the contact zones of chlorite aggregates with garnet or titanite, as well as on the faces of titanite and garnet, induction hatching.
In most cases there is a sharp boundary between the host rock and “brushes” of minerals growing on the walls of fractures and voids in these rocks. This fact, as well as the results of mineralogical observations, indicate that the mineral associations described above are most likely formed as a result of hydrothermal process accompanying contact metasomatosis. At the same time, the studied samples can be attributed to four rather contrasting mineral associations: epidote-titanite-garnet (Akhmatovskaya mine); garnet-titanite-diopside (Akhmatovskaya mine); epidote-titanite-chlorite (Nikolaje-Maksimilianovskaya mine); chlorite-titanite-garnet (Praskovie-Evgenyevskaya mine). Directly paragenic minerals for titanite in them are: garnet and epidote; garnet; epidote and clinochlore; chlorite (clinochlore?) and garnet.
Sample Prv-9 (Fig.1, f) from the Perovskitovaya mine is a contact between silicate-carbonate rock and a chlorite-calcite vein. The silicate-carbonate component, which served as a basis for the isolation of garnet monofraction for U-Pd dating, has a fine-grained structure and consists mainly of gray-blueish calcite mass, in which minerals of the olivine group (presumably forsterite or monticellite), garnet (andradite-schorlomite-morimotoite series), chlorite, and humite (clino-humite or hydroxylclino-humite) are common. This rock is diagnosed as silicate marble-marl. Chlorite-humite mineralization is common in the contact zone. The vein part is composed of large-crystalline grayish-blue calcite, dark green medium-sized chlorite (probably clinochlore) of prismatic appearance and medium-crystalline dark gray magnetite.
Characteriztion of titanite composition by major elements
Based on the results of titanite study by SEM-EDS method in the composite contrast mode, the homogeneous structure of all titanite grains was established. It is possible that the absence of zonality is a characteristic feature of titanite from the studied samples. The calculated formula coefficients for titanite grains in the central (C) and edge (E) parts are presented in Table 1-3. The composition of titanite by main elements from the samples (1-19) taken in different mine workings has no principal differences among themselves and is characterized by insignificant variation of Al. For titanite sampled from the rocks of the Akhmatovskaya mine, the typical crystallochemical formula corresponds to Ca1.00(Ti0.96Al0.05Fe0.01)1.02[Si1.00O4](O). For titanite sampled from the rocks of the Nikolaje-Maksimilianovskaya and Praskovie-Evgenyevskaya mines – Ca1.03(Ti0.95Al0.04Fe0.01)1.00[Si1.00O4](O) and Ca1.00(Ti0.95Al0.05Fe0.02)1.02[Si1.00O4](O), respectively.
Table 1
Composition and temperature of titanite formation from mineral aggregates of Akhmatovskaya mine
|
Component |
Akhm-12 |
851/7 |
||||||||||
|
1-C |
1-E |
2-C |
2-E |
3-C |
3-E |
4-C |
4-E |
5-C |
5-E |
6-C |
6-E |
|
|
Oxides of major elements, wt.% |
||||||||||||
|
SiO2 |
30.11 |
30.27 |
30.37 |
30.16 |
29.87 |
30.70 |
30.68 |
30.07 |
29.72 |
29.64 |
29.67 |
30.61 |
|
TiO2 |
38.81 |
38.11 |
37.88 |
38.52 |
39.20 |
38.45 |
38.97 |
39.59 |
38.48 |
38.83 |
39.76 |
38.22 |
|
Al2O3 |
1.67 |
1.70 |
1.69 |
1.54 |
1.59 |
1.46 |
1.27 |
1.20 |
1.29 |
1.38 |
1.32 |
1.89 |
|
FeO |
0.68 |
0.58 |
0.48 |
0.62 |
0.55 |
0.50 |
0.47 |
0.61 |
0.61 |
0.45 |
0.29 |
0.49 |
|
CaO |
28.73 |
29.33 |
29.09 |
29.16 |
28.79 |
28.90 |
28.60 |
28.52 |
28.74 |
29.70 |
28.96 |
28.78 |
|
∑com |
100.00 |
99.99 |
99.51 |
100.00 |
100.00 |
100.01 |
99.99 |
99.99 |
98.84 |
100.00 |
100.00 |
99.99 |
|
Formula coefficients (О = 5) |
||||||||||||
|
Si |
0.98 |
0.99 |
1.00 |
0.99 |
0.98 |
1.00 |
1.00 |
0.98 |
0.98 |
0.97 |
0.97 |
1.00 |
|
Ti |
0.95 |
0.94 |
0.94 |
0.95 |
0.96 |
0.94 |
0.96 |
0.97 |
0.96 |
0.96 |
0.98 |
0.94 |
|
Al |
0.06 |
0.07 |
0.07 |
0.06 |
0.06 |
0.06 |
0.05 |
0.05 |
0.05 |
0.05 |
0.05 |
0.07 |
|
Fe |
0.02 |
0.02 |
0.01 |
0.02 |
0.02 |
0.01 |
0.01 |
0.02 |
0.02 |
0.01 |
0.01 |
0.01 |
|
Ca |
1.01 |
1.03 |
1.02 |
1.02 |
1.01 |
1.01 |
1.00 |
1.00 |
1.02 |
1.04 |
1.01 |
1.00 |
|
∑cations |
3.02 |
3.05 |
3.04 |
3.04 |
3.03 |
3.02 |
3.02 |
3.02 |
3.03 |
3.03 |
3.02 |
3.02 |
|
Trace elements, ppm |
||||||||||||
|
V |
306 |
308 |
292 |
504 |
147 |
147 |
461 |
384 |
371 |
349 |
555 |
404 |
|
Cr |
21.6 |
47.6 |
21.6 |
18.0 |
27.8 |
24.7 |
14.2 |
13.5 |
35.2 |
25.2 |
13.8 |
22.3 |
|
Sr |
82.8 |
89.2 |
82.4 |
88.8 |
92.8 |
94.1 |
88.8 |
87.0 |
92.9 |
87.4 |
80.4 |
79.0 |
|
Y |
10.5 |
9.9 |
10.1 |
10.2 |
12.7 |
11.6 |
30.6 |
32.5 |
11.8 |
11.1 |
20.2 |
13.2 |
|
Zr |
225 |
223 |
219 |
234 |
373 |
376 |
739 |
511 |
330 |
256 |
724 |
292 |
|
Nb |
152 |
132 |
159 |
158 |
221 |
220 |
110 |
143 |
255 |
174 |
719 |
199 |
|
Ba |
2.28 |
2.56 |
1.90 |
2.59 |
4.15 |
4.06 |
5.41 |
4.47 |
5.28 |
5.43 |
2.97 |
3.99 |
|
La |
11.3 |
11.0 |
11.3 |
11.9 |
11.2 |
10.5 |
82.3 |
62.5 |
12.6 |
9.5 |
16.6 |
10.6 |
|
Ce |
51.0 |
59.6 |
62.4 |
66.9 |
80.0 |
77.1 |
433 |
318 |
84.1 |
70.8 |
136 |
75.5 |
|
Pr |
11.4 |
13.0 |
14.6 |
14.8 |
26.7 |
25.6 |
99.5 |
78.1 |
27.6 |
22.5 |
41.7 |
26.6 |
|
Nd |
63.3 |
73.6 |
86.1 |
91.7 |
207 |
201 |
622 |
495 |
224 |
185 |
334 |
214 |
|
Sm |
15.5 |
19.0 |
20.0 |
23.1 |
51.8 |
55.3 |
109 |
99.3 |
54.5 |
54.4 |
104 |
52.8 |
|
Eu |
5.90 |
6.35 |
7.43 |
7.89 |
15.6 |
16.2 |
29.0 |
27.2 |
16.1 |
15.3 |
27.9 |
16.6 |
|
Gd |
6.10 |
10.1 |
9.01 |
10.9 |
24.9 |
22.5 |
41.8 |
32.3 |
21.9 |
18.9 |
27.3 |
24.0 |
|
Dy |
3.43 |
3.38 |
4.05 |
4.16 |
8.79 |
8.12 |
20.0 |
18.0 |
8.11 |
7.88 |
14.5 |
8.96 |
|
Er |
0.56 |
0.71 |
0.46 |
0.91 |
1.13 |
1.03 |
3.34 |
2.53 |
1.91 |
1.27 |
2.29 |
1.42 |
|
Yb |
0.46 |
0.34 |
0.40 |
0.38 |
1.89 |
1.19 |
2.41 |
2.63 |
1.75 |
0.63 |
1.84 |
1.12 |
|
Lu |
0.16 |
0.12 |
0.17 |
0.20 |
0.31 |
0.32 |
0.74 |
0.75 |
0.31 |
0.25 |
0.59 |
0.34 |
|
Hf |
7.69 |
7.03 |
6.98 |
6.21 |
11.2 |
12.2 |
18.0 |
13.0 |
9.67 |
7.25 |
21.3 |
8.53 |
|
Ta |
4.15 |
3.15 |
3.21 |
3.01 |
5.51 |
5.31 |
2.38 |
3.53 |
6.84 |
4.60 |
14.1 |
5.42 |
|
Th |
0.03 |
0.05 |
0.02 |
0.08 |
0.29 |
0.20 |
1.72 |
1.52 |
0.29 |
0.24 |
0.71 |
0.24 |
|
U |
0.17 |
0.13 |
0.13 |
0.17 |
1.36 |
1.10 |
1.83 |
1.75 |
1.20 |
1.29 |
3.60 |
1.41 |
|
ΣREE |
169 |
197 |
216 |
233 |
429 |
419 |
1,444 |
1,137 |
453 |
387 |
707 |
432 |
|
ΣLa-Nd |
137 |
157 |
174 |
185 |
325 |
314 |
1,237 |
954 |
348 |
288 |
528 |
327 |
|
ΣGd-Lu |
10.7 |
14.6 |
14.1 |
16.5 |
37.0 |
33.2 |
68.3 |
56.2 |
34.0 |
29.0 |
46.5 |
35.9 |
|
LREE/HREE |
12.8 |
10.7 |
12.4 |
11.2 |
8.78 |
9.48 |
18.1 |
17.0 |
10.3 |
9.93 |
11.4 |
9.11 |
|
Се/Се* |
1.08 |
1.20 |
1.18 |
1.22 |
1.12 |
1.14 |
1.16 |
1.10 |
1.09 |
1.17 |
1.25 |
1.09 |
|
Eu/Eu* |
1.85 |
1.40 |
1.69 |
1.52 |
1.32 |
1.40 |
1.30 |
1.46 |
1.42 |
1.45 |
1.59 |
1.42 |
|
Zr/Hf |
29.3 |
31.7 |
31.4 |
37.7 |
33.1 |
30.9 |
41.0 |
39.4 |
34.2 |
35.3 |
33.9 |
34.2 |
|
Nb/Ta |
36.6 |
42.0 |
49.5 |
52.3 |
40.2 |
41.3 |
46.1 |
40.6 |
37.2 |
37.8 |
51.1 |
36.6 |
|
Th/U |
0.17 |
0.40 |
0.13 |
0.46 |
0.21 |
0.18 |
0.94 |
0.87 |
0.24 |
0.19 |
0.20 |
0.17 |
|
Formation temperature of titanite |
||||||||||||
|
Т, °С |
682 |
682 |
681 |
685 |
709 |
709 |
747 |
726 |
702 |
689 |
746 |
696 |
Table 2
Composition and temperature of titanite formation from mineral aggregates of the Nikolaje-Maksimilianovskaya mine
|
Component |
851/34 |
|||||||||
|
7-C |
7-E |
8-C |
8-E |
9-C |
9-E |
10-C |
10-E |
11-C |
11-E |
|
|
Oxides of major elements, wt.% |
||||||||||
|
SiO2 |
31.54 |
29.28 |
30.33 |
30.57 |
30.01 |
30.34 |
29.83 |
29.31 |
29.90 |
29.65 |
|
TiO2 |
38.52 |
39.32 |
39.45 |
38.57 |
39.57 |
38.77 |
39.87 |
38.82 |
38.06 |
39.61 |
|
Al2O3 |
1.53 |
1.04 |
1.05 |
1.13 |
0.80 |
1.60 |
0.92 |
1.40 |
2.01 |
1.13 |
|
FeO |
0.56 |
0.32 |
0.54 |
0.36 |
0.55 |
0.61 |
0.41 |
0.52 |
0.80 |
0.44 |
|
CaO |
27.85 |
28.85 |
28.62 |
29.37 |
29.07 |
28.67 |
28.66 |
28.29 |
29.12 |
29.17 |
|
∑com |
100.00 |
98.81 |
99.99 |
100.00 |
100.00 |
99.99 |
99.69 |
98.34 |
99.89 |
100.00 |
|
Formula coefficients (О = 5) |
||||||||||
|
Si |
1.02 |
0.97 |
0.99 |
1.00 |
0.98 |
0.99 |
0.98 |
0.97 |
0.98 |
0.97 |
|
Ti |
0.94 |
0.98 |
0.97 |
0.95 |
0.97 |
0.95 |
0.98 |
0.97 |
0.94 |
0.98 |
|
Al |
0.06 |
0.04 |
0.04 |
0.04 |
0.03 |
0.06 |
0.04 |
0.05 |
0.08 |
0.04 |
|
Fe |
0.02 |
0.01 |
0.01 |
0.01 |
0.02 |
0.02 |
0.01 |
0.01 |
0.02 |
0.01 |
|
Ca |
0.97 |
1.02 |
1.00 |
1.03 |
1.02 |
1.00 |
1.01 |
1.01 |
1.02 |
1.02 |
|
∑cations |
3.01 |
3.02 |
3.01 |
3.03 |
3.02 |
3.02 |
3.02 |
3.01 |
3.04 |
3.02 |
|
Trace elements, ppm |
||||||||||
|
V |
613 |
365 |
569 |
1255 |
478 |
460 |
514 |
553 |
402 |
709 |
|
Cr |
20.3 |
31.0 |
23.4 |
12.4 |
27.6 |
29.0 |
24.0 |
23.4 |
21.9 |
20.6 |
|
Sr |
102 |
87.5 |
79.5 |
85.9 |
98.8 |
106 |
96.5 |
95.7 |
94.6 |
93.3 |
|
Y |
340 |
527 |
424 |
215 |
390 |
432 |
322 |
311 |
576 |
444 |
|
Zr |
418 |
240 |
296 |
226 |
357 |
570 |
314 |
334 |
253 |
199 |
|
Nb |
186 |
442 |
313 |
184 |
217 |
519 |
282 |
242 |
417 |
165 |
|
Ba |
4.80 |
6.65 |
4.30 |
2.56 |
17.2 |
3.53 |
2.64 |
2.64 |
2.84 |
2.73 |
|
La |
0.59 |
0.43 |
0.40 |
0.80 |
0.15 |
0.58 |
0.16 |
0.17 |
0.42 |
0.24 |
|
Ce |
1.58 |
2.57 |
2.91 |
0.61 |
0.65 |
3.16 |
0.83 |
0.84 |
2.43 |
1.43 |
|
Pr |
0.61 |
0.99 |
1.00 |
0.51 |
0.37 |
1.25 |
0.36 |
0.35 |
0.92 |
0.54 |
|
Nd |
4.46 |
8.35 |
10.4 |
2.44 |
3.53 |
9.89 |
2.84 |
2.54 |
7.98 |
6.20 |
|
Sm |
4.11 |
7.86 |
10.6 |
3.41 |
5.75 |
9.50 |
3.26 |
3.50 |
10.1 |
8.84 |
|
Eu |
4.53 |
5.19 |
5.78 |
1.35 |
4.96 |
5.48 |
2.54 |
2.59 |
6.33 |
6.69 |
|
Gd |
11.9 |
19.0 |
22.7 |
9.4 |
16.3 |
20.0 |
8.4 |
8.4 |
23.6 |
24.2 |
|
Dy |
35.7 |
61.3 |
53.8 |
28.6 |
47.7 |
53.4 |
31.3 |
30.8 |
69.9 |
62.0 |
|
Er |
39.0 |
51.9 |
41.8 |
25.7 |
39.6 |
40.7 |
34.1 |
34.7 |
52.9 |
41.1 |
|
Yb |
45.8 |
51.3 |
41.5 |
29.0 |
41.7 |
45.0 |
46.9 |
42.0 |
54.3 |
35.1 |
|
Lu |
4.78 |
5.93 |
4.48 |
2.81 |
4.35 |
5.16 |
4.39 |
4.70 |
6.12 |
4.18 |
|
Hf |
31.7 |
27.5 |
30.6 |
22.4 |
31.1 |
34.7 |
26.9 |
26.2 |
36.7 |
26.1 |
|
Ta |
8.28 |
22.5 |
13.8 |
8.39 |
10.3 |
21.2 |
9.81 |
8.27 |
21.3 |
11.6 |
|
Th |
0.03 |
0.06 |
0.09 |
0.07 |
0.02 |
0.06 |
0.03 |
0.05 |
0.09 |
0.05 |
|
U |
1.02 |
0.82 |
0.80 |
0.09 |
0.74 |
0.97 |
0.37 |
0.51 |
1.17 |
1.01 |
|
ΣREE |
60.0 |
76.1 |
38.3 |
35.6 |
24.4 |
9.2 |
16.9 |
8.40 |
32.8 |
31.1 |
|
ΣLa-Nd |
15.0 |
17.9 |
8.73 |
9.50 |
5.42 |
2.54 |
4.18 |
2.61 |
9.61 |
7.38 |
|
ΣGd-Lu |
35.1 |
46.0 |
23.2 |
20.9 |
14.7 |
5.17 |
9.85 |
4.09 |
16.9 |
18.0 |
|
LREE/HREE |
0.43 |
0.39 |
0.38 |
0.45 |
0.37 |
0.49 |
0.42 |
0.64 |
0.57 |
0.41 |
|
Се/Се* |
0.63 |
0.96 |
1.11 |
0.23 |
0.67 |
0.90 |
0.83 |
0.82 |
0.95 |
0.96 |
|
Eu/Eu* |
1.97 |
1.29 |
1.13 |
0.73 |
1.56 |
1.21 |
1.48 |
1.46 |
1.25 |
1.40 |
|
Zr/Hf |
14.6 |
14.2 |
8.9 |
13.0 |
13.9 |
23.4 |
21.0 |
23.8 |
14.7 |
10.9 |
|
Nb/Ta |
40.1 |
42.5 |
42.8 |
36.7 |
19.6 |
37.4 |
34.4 |
27.6 |
31.8 |
16.8 |
|
Th/U |
0.61 |
0.46 |
0.65 |
0.57 |
0.33 |
0.59 |
0.31 |
0.75 |
1.45 |
0.67 |
|
Formation temperature of titanite |
||||||||||
|
Т, °С |
715 |
686 |
697 |
683 |
706 |
732 |
700 |
703 |
688 |
676 |
Table 3
Composition and temperature of titanite formation from mineral aggregates of Praskovie-Evgenyevskaya mine
|
Component |
851/42 |
851/44 |
||||||||||||||
|
12-C |
12-E |
13-C |
13- E |
14-C |
14- E |
15-C |
15- E |
16-C |
16- E |
17-C |
17- E |
18-C |
18- E |
19-C |
19- E |
|
|
Oxides of major elements, wt.% |
||||||||||||||||
|
SiO2 |
30.69 |
30.11 |
30.57 |
30.10 |
30.03 |
30.02 |
30.64 |
30.83 |
30.20 |
30.11 |
30.04 |
30.43 |
30.96 |
31.04 |
30.61 |
30.81 |
|
TiO2 |
39.96 |
39.34 |
38.89 |
39.75 |
38.77 |
39.22 |
38.83 |
38.85 |
39.33 |
39.19 |
39.39 |
39.37 |
38.52 |
38.80 |
39.73 |
38.94 |
|
Al2O3 |
0.98 |
0.87 |
1.13 |
1.21 |
1.14 |
1.18 |
1.23 |
1.16 |
1.05 |
0.81 |
1.11 |
0.78 |
1.22 |
1.09 |
0.84 |
0.88 |
|
FeO |
0.24 |
0.67 |
0.49 |
0.39 |
0.57 |
0.53 |
0.59 |
0.72 |
0.57 |
0.42 |
0.42 |
0.39 |
0.48 |
0.46 |
0.19 |
0.36 |
|
CaO |
28.97 |
29.01 |
28.93 |
28.54 |
29.50 |
29.05 |
28.71 |
28.44 |
28.84 |
29.48 |
29.04 |
29.03 |
28.82 |
28.60 |
28.63 |
29.00 |
|
∑com |
100.84 |
100.00 |
100.01 |
99.99 |
100.01 |
100.00 |
100.00 |
100.00 |
99.99 |
100.01 |
100.00 |
100.00 |
100.00 |
99.99 |
100.00 |
99.99 |
|
Formula coefficients (О = 5) |
||||||||||||||||
|
Si |
0.99 |
0.99 |
1.00 |
0.98 |
0.98 |
0.98 |
1.00 |
1.01 |
0.99 |
0.99 |
0.98 |
1.00 |
1.01 |
1.01 |
1.00 |
1.01 |
|
Ti |
0.97 |
0.97 |
0.95 |
0.98 |
0.96 |
0.97 |
0.95 |
0.95 |
0.97 |
0.97 |
0.97 |
0.97 |
0.95 |
0.95 |
0.97 |
0.96 |
|
Al |
0.04 |
0.03 |
0.04 |
0.05 |
0.04 |
0.05 |
0.05 |
0.04 |
0.04 |
0.03 |
0.04 |
0.03 |
0.05 |
0.04 |
0.03 |
0.03 |
|
Fe |
0.01 |
0.02 |
0.01 |
0.01 |
0.02 |
0.01 |
0.02 |
0.02 |
0.02 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
|
Ca |
1.00 |
1.02 |
1.01 |
1.00 |
1.04 |
1.02 |
1.00 |
0.99 |
1.01 |
1.03 |
1.02 |
1.02 |
1.01 |
1.00 |
1.00 |
1.01 |
|
∑cations |
3.01 |
3.03 |
3.01 |
3.02 |
3.04 |
3.03 |
3.02 |
3.01 |
3.03 |
3.03 |
3.02 |
3.03 |
3.03 |
3.01 |
3.01 |
3.02 |
|
Trace elements, ppm |
||||||||||||||||
|
V |
3,886 |
3,773 |
3,978 |
3,837 |
3,435 |
3,619 |
4,048 |
3,796 |
3,905 |
3,211 |
3,617 |
3,299 |
3,599 |
3,699 |
3,046 |
3,553 |
|
Cr |
177 |
159 |
173 |
140 |
143 |
139 |
154 |
139 |
138 |
110 |
115 |
106 |
107 |
97.9 |
138 |
115 |
|
Sr |
142 |
157 |
135 |
136 |
134 |
130 |
118 |
111 |
123 |
106 |
110 |
112 |
115 |
115 |
106 |
113 |
|
Y |
74.8 |
96.0 |
50.0 |
42.2 |
35.3 |
14.0 |
20.1 |
10.6 |
30.4 |
39.8 |
16.5 |
43.7 |
21.3 |
18.0 |
28.4 |
21.2 |
|
Zr |
63.3 |
97.1 |
24.2 |
31.6 |
38.0 |
17.0 |
23.7 |
19.3 |
32.8 |
21.5 |
23.8 |
27.9 |
18.9 |
21.6 |
17.5 |
27.1 |
|
Nb |
109 |
142.6 |
49.0 |
57.2 |
14.9 |
8.48 |
19.8 |
6.08 |
28.9 |
16.0 |
16.9 |
39.7 |
19.9 |
21.4 |
13.5 |
43.0 |
|
Ba |
48.0 |
22.0 |
1.43 |
1.12 |
1.35 |
2.53 |
2.59 |
2.06 |
3.86 |
6.63 |
4.69 |
3.24 |
3.20 |
4.10 |
3.33 |
2.47 |
|
La |
0.51 |
0.56 |
0.25 |
0.31 |
0.14 |
0.10 |
0.10 |
0.07 |
0.29 |
0.22 |
0.16 |
0.31 |
0.12 |
0.16 |
0.23 |
0.17 |
|
Ce |
3.43 |
4.37 |
1.93 |
2.29 |
1.13 |
0.52 |
0.97 |
0.58 |
2.59 |
1.56 |
0.92 |
2.38 |
0.73 |
0.89 |
1.69 |
1.14 |
|
Pr |
1.12 |
1.34 |
0.72 |
0.75 |
0.40 |
0.18 |
0.29 |
0.17 |
0.74 |
0.59 |
0.33 |
1.04 |
0.26 |
0.26 |
0.65 |
0.40 |
|
Nd |
9.95 |
11.6 |
5.83 |
6.15 |
3.74 |
1.73 |
2.81 |
1.78 |
6.00 |
5.01 |
2.96 |
8.72 |
2.25 |
2.41 |
5.81 |
3.65 |
|
Sm |
5.42 |
6.73 |
3.54 |
3.00 |
2.35 |
0.92 |
1.73 |
1.09 |
4.05 |
3.39 |
1.70 |
3.82 |
1.40 |
1.48 |
2.74 |
1.80 |
|
Eu |
4.42 |
5.43 |
2.86 |
2.19 |
1.87 |
0.59 |
1.13 |
0.61 |
2.22 |
2.33 |
1.06 |
2.88 |
1.06 |
1.03 |
2.42 |
1.23 |
|
Gd |
6.97 |
9.29 |
4.92 |
4.05 |
3.23 |
1.17 |
1.80 |
1.00 |
4.88 |
4.41 |
1.82 |
4.63 |
1.90 |
1.50 |
3.73 |
1.89 |
|
Dy |
10.1 |
12.5 |
6.17 |
5.13 |
4.24 |
1.33 |
2.30 |
1.00 |
4.29 |
6.17 |
2.19 |
5.97 |
2.59 |
2.02 |
4.61 |
2.25 |
|
Er |
6.86 |
9.35 |
4.73 |
4.07 |
3.15 |
1.13 |
1.90 |
0.80 |
3.46 |
3.77 |
1.29 |
3.25 |
2.08 |
1.79 |
2.42 |
1.75 |
|
Yb |
9.93 |
13.1 |
6.41 |
6.68 |
3.62 |
1.32 |
3.39 |
1.13 |
3.79 |
3.13 |
1.88 |
4.46 |
2.62 |
2.84 |
2.42 |
3.95 |
|
Lu |
1.31 |
1.76 |
0.92 |
0.96 |
0.49 |
0.21 |
0.45 |
0.16 |
0.49 |
0.48 |
0.28 |
0.70 |
0.38 |
0.35 |
0.45 |
0.53 |
|
Hf |
4.35 |
6.84 |
2.71 |
2.42 |
2.74 |
0.73 |
1.13 |
0.81 |
2.23 |
1.97 |
1.30 |
3.79 |
0.93 |
0.97 |
1.54 |
1.38 |
|
Ta |
2.71 |
3.35 |
1.14 |
1.56 |
0.76 |
0.23 |
0.58 |
0.22 |
0.91 |
0.95 |
0.58 |
1.70 |
0.48 |
0.62 |
0.99 |
1.30 |
|
Th |
0.22 |
0.27 |
0.11 |
0.11 |
0.02 |
0.03 |
0.04 |
0.02 |
0.16 |
0.08 |
0.11 |
0.34 |
0.03 |
0.09 |
0.15 |
0.13 |
|
U |
0.35 |
0.59 |
0.18 |
0.19 |
0.06 |
0.05 |
0.13 |
0.02 |
0.11 |
0.13 |
0.10 |
0.20 |
0.07 |
0.13 |
0.14 |
0.29 |
|
ΣREE |
14.6 |
38.2 |
15.4 |
14.7 |
27.2 |
18.8 |
153.1 |
214.9 |
195 |
105 |
165 |
194 |
135 |
131 |
235 |
191 |
|
ΣLa-Nd |
4.37 |
12.5 |
3.36 |
3.73 |
8.38 |
5.35 |
7.25 |
12.3 |
14.7 |
4.37 |
4.69 |
14.9 |
4.20 |
3.90 |
11.8 |
8.42 |
|
ΣGd-Lu |
7.46 |
19.0 |
9.57 |
8.50 |
13.6 |
10.4 |
137.2 |
189.5 |
164 |
95.5 |
150 |
164 |
125 |
121 |
207 |
167 |
|
LREE/HREE |
0.59 |
0.65 |
0.35 |
0.44 |
0.62 |
0.52 |
0.05 |
0.07 |
0.09 |
0.05 |
0.03 |
0.09 |
0.03 |
0.03 |
0.06 |
0.05 |
|
Се/Се* |
1.10 |
1.22 |
1.10 |
1.14 |
1.14 |
0.94 |
1.36 |
1.24 |
1.36 |
1.04 |
0.96 |
1.01 |
0.99 |
1.06 |
1.06 |
1.08 |
|
Eu/Eu* |
2.19 |
2.09 |
2.09 |
1.91 |
2.07 |
1.73 |
1.95 |
1.77 |
1.52 |
1.83 |
1.83 |
2.09 |
1.98 |
2.11 |
2.31 |
2.04 |
|
Zr/Hf |
18.2 |
7.35 |
20.3 |
22.2 |
11.3 |
19.6 |
13.2 |
8.74 |
9.67 |
10.1 |
11.5 |
16.5 |
11.7 |
12.7 |
6.89 |
7.62 |
|
Nb/Ta |
29.2 |
23.3 |
41.5 |
34.8 |
13.6 |
33.1 |
22.5 |
19.6 |
22.7 |
21.9 |
21.1 |
24.4 |
28.8 |
29.3 |
19.6 |
14.2 |
|
Th/U |
1.08 |
1.69 |
0.45 |
0.70 |
1.08 |
0.46 |
0.03 |
0.08 |
0.11 |
0.84 |
0.03 |
0.07 |
0.08 |
0.10 |
0.08 |
0.05 |
|
Formation temperature of titanite |
||||||||||||||||
|
Т, °С |
622 |
642 |
581 |
592 |
600 |
567 |
580 |
572 |
594 |
576 |
580 |
587 |
571 |
577 |
568 |
586 |
Characterization of titanite composition by rare elements
In contrast to the main elements, the content of rare elements in titanite varies widely. Titanite from the chlorite-titanite-garnet association (Praskovie-Evgenyevskaya mine) has an increased content of Cr, V, and Sr with a decreased concentration of Ta, Nb, Hf and Zr. Table 4 shows the median, minimum and maximum for the content of these elements in comparison with titanite from other mineral associations, which is enriched in Ta, Nb, Hf and Zr and depleted in Cr, V, and Sr. The titanite from the epidote-titanite-clinochlore association of the Nikolaje-Maksimilianovskoe mine is characterized by a significantly increased (by an order of magnitude) Y content. The content of Ba, U and Th in titanite is characterized by the least variability in comparison with other elements.
Table 4
Values of some statistical parameters for rare elements in the composition of titanite
|
Mine |
Association |
Parameters |
V |
Cr |
Sr |
Y |
Zr |
Nb |
Ba |
Hf |
Ta |
Th |
U |
|
Akhmatovskaya(n = 6) |
Epidote-titanite-garnet |
Median |
299 |
23.2 |
89.0 |
10.3 |
230 |
158 |
2.57 |
7.36 |
3.68 |
0.06 |
0.17 |
|
Minimum |
147 |
18.0 |
82.4 |
9.94 |
219 |
132 |
1.90 |
6.21 |
3.01 |
0.02 |
0.13 |
||
|
Maximum |
504 |
47.6 |
94.1 |
12.7 |
376 |
221 |
4.15 |
12.18 |
5.51 |
0.29 |
1.36 |
||
|
Garnet-titanite-diopside |
Median |
394 |
18.3 |
87.2 |
16.7 |
421 |
186 |
4.87 |
11.3 |
5.01 |
0.50 |
1.58 |
|
|
Minimum |
349 |
13.5 |
79.0 |
11.1 |
256 |
185 |
2.97 |
7.25 |
2.38 |
0.24 |
1.20 |
||
|
Maximum |
555 |
35.2 |
92.9 |
32.5 |
739 |
719 |
5.43 |
21.3 |
14.1 |
1.72 |
3.60 |
||
|
Nikolaje-Maksimilianovskaya(n = 10) |
Epidote-titanite-chlorite |
Median |
534 |
23.4 |
95.1 |
407 |
305 |
262 |
3.18 |
29.0 |
10.9 |
0.06 |
0.81 |
|
Minimum |
365 |
12.4 |
79.5 |
215 |
199 |
165 |
2.56 |
22.4 |
8.27 |
0.02 |
0.09 |
||
|
Maximum |
1,255 |
31.0 |
106 |
576 |
570 |
519 |
17.2 |
36.7 |
22.5 |
0.09 |
1.17 |
||
|
Praskovie-Evgenyevskaya (n = 8) |
Chlorite-titanite-garnet (little garnet) |
Median |
3,816 |
149 |
135 |
38.8 |
27.9 |
34.4 |
2.29 |
2.57 |
0.95 |
0.07 |
0.15 |
|
Minimum |
3,435 |
139 |
111 |
10.6 |
17.0 |
6.08 |
1.12 |
0.73 |
0.22 |
0.02 |
0.02 |
||
|
Maximum |
4,048 |
177 |
157 |
96.0 |
97.1 |
143 |
48.0 |
6.84 |
3.35 |
0.27 |
0.59 |
||
|
Chlorite-titanite-garnet (garnet prevails) |
Median |
3,576 |
113 |
112 |
24.8 |
22.7 |
20.7 |
3.59 |
1.46 |
0.93 |
0.12 |
0.13 |
|
|
Minimum |
3,046 |
97.9 |
106 |
16.5 |
17.5 |
13.5 |
2.47 |
0.93 |
0.48 |
0.03 |
0.07 |
||
|
Maximum |
3,905 |
138 |
123 |
43.7 |
32.8 |
43.0 |
6.63 |
3.79 |
1.70 |
0.34 |
0.29 |
Figurative points of titanite on the binary diagram in coordinates of V and Cr contents in (Fig.2, a) are arranged in the form of two discrete groups. One group (compact arrangement of points) is titanite from chlorite-titanite-garnet association of Praskovie-Evgenyevskaya mine with V and Cr content not less than 3,000 and 100 ppm, respectively. Another (scattered cluster of points) is titanite from mineral aggregates of Akhmatovskaya and Nikolaje-Maksimilianovskaya mines with V content from 100 to 1,000 ppm and Cr content from 10 to 50 ppm. At the same time for titanite from Nikolaje-Maksimilianovskaya mine negative correlation between these elements is observed.
Titanite from all objects shows a strong positive correlation between the contents of Nb and Ta, geochemically close to each other highly charged elements (Fig.2, b). It should be noted that titanite from crystalline aggregates sampled in the Praskovie-Evgenyevskaya mine is in the lower part of this trend, differing by a reduced content of Nb (not more than 100 ppm) and Ta (not more than 3 ppm) from titanite in other objects. The maximum content of these elements is characteristic of titanite from the epidote-titanite-clinochlore association of the Nikolaje-Maksimilianovskaya mine.
The ratio of another pair of highly charged elements (Zr and Hf) in titanite also shows a positive relationship with each other, while dividing into three independent clusters (Fig.2, c). Titanite from mineral aggregates of Praskovie-Evgenyevskaya mine has the lowest contents of Zr (for the majority, not more than 40 ppm) and Hf (mainly, not more than 4 ppm) in comparison with titanite from other objects. At the same time, in the association where garnet is minimal, the marginal parts of titanite crystals (average, 24.5 and 2.03 ppm), compared to the central parts (average, 23.2 and 1.50 ppm), are found to be more enriched in Zr and Hf than in the garnet-dominated association (the only significant case of difference). The maximum Zr content is noted in titanite from crystalline aggregates of the Akhmatovskaya mine and ranges from 200 to 1,000 ppm, with Hf concentrations ranging from 6 to 20 ppm. The highest Hf content (from 20 to 40 ppm) is characteristic of titanite from the epidote-titanite-clinochlore association of the Nikolaje-Maksimilianovskaya mine, the Zr content in which varies from 200 to 600 ppm.
The Ba and Y contents do not demonstrate the dependence between these elements, although they reflect a significant difference in the composition of titanite from the aggregates sampled in the Nikolaje-Maksimilianovskaya mine from titanite sampled in other objects (Fig.2, d). The highest Y content was found in titanite (from 200 to 600 ppm) from epidote-titanite-clinochlore aggregate (Nikolaje-Maksimilianovskaya mine). The concentration of Y in titanite from other objects is in the range from 10 to 100 ppm. For most of the studied titanite, Ba content does not exceed 10 ppm. The sharp variation of Ba content in titanite from chlorite-titanite-garnet association (Praskovie-Evgenyevskaya mine) can be related to its inheritance from previously formed protolith minerals (e.g., K-Na feldspars or mica of phlogopite-annite series).
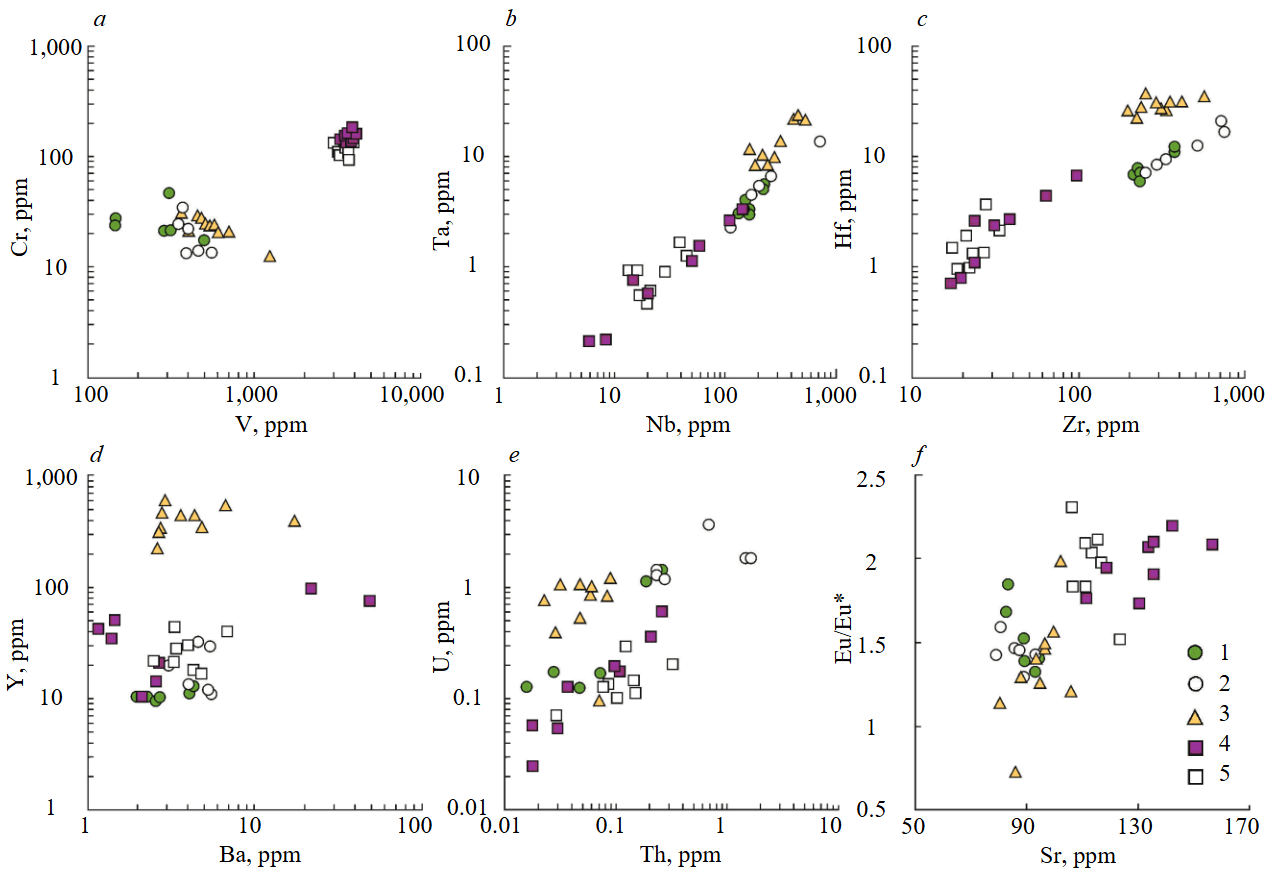
Fig.2. Binary diagrams of the ratio of trace elements V и Cr (а), Nb и Ta (b), Zr и Hf (c), Ba и Y (d), Th и U (e), Sr and Eu/Eu*(f) in titanite from different mineral associations
1 – epidote-titanite-garnet (sam. Akhm-12, Akhmatovskaya mine); 2 – garnet-titanite-diopside (sam. 851/7, Akhmatovskaya mine); 3 – epidote-titanite-clinochlore (sam. 851/34, Nikolaje-Maksimilianovskaya mine); 4, 5 – chlorite-titanite-garnet (Praskovie-Evgenyevskaya mine): 4 – garnet in subordinate quantity (sam. 851/42); 5 – garnet prevails (sam. 851/44)
Th and U contents in the composition of all titanite grains show weak positive correlation and, as a rule, do not exceed 2 ppm (Fig.2, e). The highest, relative to other objects, contents of these elements were found for titanite from garnet-titanite-diopside association in the Akhmatovskaya mine. Titanite from the epidote-titanite-garnet association from the same mine is characterized by noticeably lower Th and U contents, which is probably related to the incorporation of these elements into epidote. Titanite from the epidote-titanite-clinochlore association of the Nikolaje-Maksimilianovskaya mine is characterized by increased U content with moderate Th content.
There is a weak positive correlation between the Sr content in titanite and the amplitude of its Eu-anomaly, indicating the isomorphic occurrence of Sr and Eu in the Ca position (Fig.2, f). Titanite from mineral aggregates of the Praskovie-Evgenyevskaya mine is characterized by the maximum Sr content, correlating with the largest amplitude of positive Eu-anomaly (Eu/Eu* from 1.5 to 2.3). The Sr concentration in titanite, in association with which garnet occurs sporadically, is predominantly in the range from 130 to 170 ppm, while in titanite, in association with which garnet predominates, – from 100 to 120 ppm. The Sr content for the majority of titanite grains from crystalline aggregates sampled in the Akhmatovskaya and Nikolaje-Maksimilianovskaya mines is mainly in the range from 80 to 120 ppm, with Eu/Eu* from 1.2 to 1.6.
Characterization of titanite composition by rare-earth elements
The REE content in the considered titanite crystals also demonstrates a clear difference in the composition of titanite from one mineralogical mine relative to the composition of titanite from other mines. Titanite from mineral aggregates of Akhmatovskaya mine is characterized by the highest REE enrichment – on the average, the total REE content is (hereinafter medians are given) 518 ppm, LREE/HREE ratio is 11.7. At the same time, titanite from the garnet-titanite-diopside association is characterized by increased REE content (total REE – 580 ppm, LREE/HREE – 10.8), relative to titanite from the epidote-titanite-garnet association (total REE – 224 ppm, LREE/HREE – 10.9). The REE distribution spectra for titanite from the rocks of the Akhmatovskaya mine are similar to each other and have a convex shape in the LREE region and a concave shape in the HREE region (Fig.3, a). In general, REE spectra are strongly differentiated with predominance of LREE over HREE. In addition, all REE distribution spectra of the considered titanite show a positive Eu-anomaly, for which Eu/Eu* is 1.49, while Ce-anomaly is practically not manifested (Ce/Ce* is 1.15).
The REE content in titanite from epidote-titanite-clinochlore association of the Nikolaje-Maksimilianovskaya mine is 31.9 ppm, with the LREE/HREE ratio of 0.43 (Fig.3, b). The character of REE distribution spectra, relative to titanite from other objects, is characterized by the lowest REE differentiation, sharp positive slope and convex character in the HREE region. A positive Eu-anomaly (Eu/Eu* is 1.40, except for one spectrum with a value of 0.73), and absence of Ce-anomaly (Ce/Ce* is 0.95, except for three analyses, in which Ce/Ce* is 0.63, 0.67 and 0.23) are established for the majority of titanite grains.
For titanite from mineral aggregates sampled in Praskovie-Evgenyevskaya mine REE content –133 ppm, LREE/HREE – 0.08 (Fig.3, c). It should be noted that for titanite, in association with which garnet predominates, an increased REE content (total REE – 178 ppm, LREE/HREE – 0.05) was established with a narrow range of REE distribution spectra. Whereas titanite with which garnet has a subordinate role is characterized by a reduced REE content (total REE – 23.0 ppm, LREE/HREE – 0.48) accross a wide range of REE distribution spectra. In general, the REE distribution spectra are characterized by a similar conformal character with a convex shape of the spectrum in the LREE region and a relatively hollow shape in the HREE region. All REE distribution spectra show a clear positive Eu-anomaly (Eu/Eu* is 2.01) and no Ce-anomaly (Ce/Ce* is 1.09).
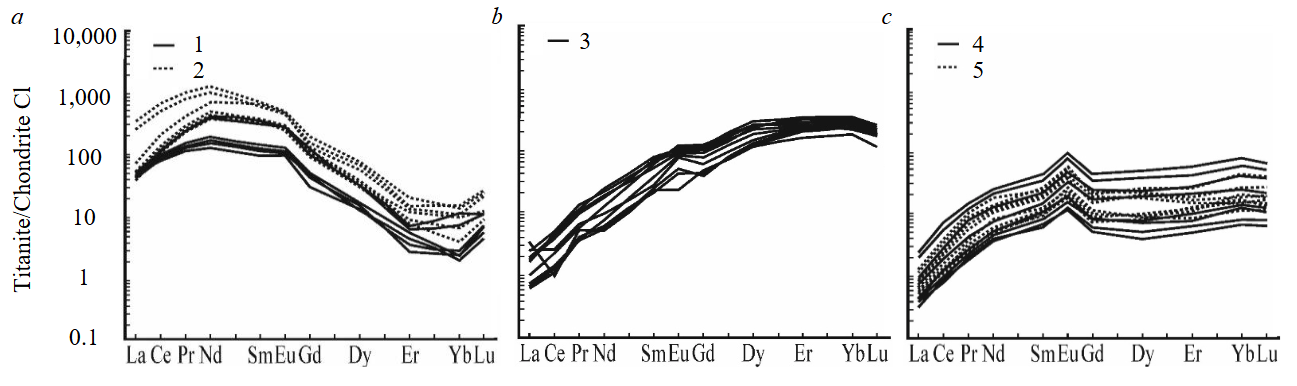
Fig.3. Distribution spectra of rare-earth elements in titanite from epidote-titanite-garnet and garnet-titanite-diopside (a); epidote-titanite-clinochlore (b); chlorite-titanite-garnet (c) mineral associations
1 – sam. Аkhm-12; 2 – sam. 851/7; 3 – sam. 851/34; 4 – sam. 851/42 (garnet in a subordinate quantity); 5 – sam. 851/44 (garnet prevails)
U-Pb age of garnet
Five analyses were performed for garnets from silicate-carbonate rocks sampled in the Perovskitovaya mine. The Pb content of the garnet ranges from 1.6 to 2.1 ppm, the U content from 21.7 to 28.5 ppm. The high 206Pb/204Pb isotopic ratios (1,055 to 6,500) reflect the low content of common Pb. The Th/U ratio less than unity (Table 5), calculated from the ratio (208Pb/206Pb)rad taking into account the age, indicates the absence of inclusions of other minerals in the garnet. In Table 5 all errors correspond to the 2σ level, the error values correspond to the last significant digits.
Table 5
U-Pb isotopic data for garnets from silicate-carbonate rocks of the Perovskitovaya mine
|
Processing |
Weight, mg |
Pb, mkg/g |
U, mkg/g |
Pbc /Pbt |
Th/U** |
206Pb/204Pb*** |
|||||||||
|
3.3N HCl (1)* |
6.93 |
1.600 |
21.743 |
0.009 |
0.02 |
6,500 |
|||||||||
|
3.3N HCl (3) |
11.82 |
1.872 |
25.30 |
0.013 |
0.02 |
5,012 |
|||||||||
|
3.3N HCl (2) |
9.13 |
2.007 |
26.9 |
0.021 |
0.02 |
3,200 |
|||||||||
|
2N HCl (3) |
18.52 |
1.906 |
24.29 |
0.062 |
0.02 |
1,055 |
|||||||||
|
2N HCl (3) |
19.92 |
2.135 |
28.52 |
0.02 |
0.02 |
3,417 |
|||||||||
|
Processing |
Isotopic ratios corrected for mass fractionation, blank experiment and conventional Pb |
Rho**** |
Age, Ma |
||||||||||||
|
207Pb/206Pb |
208Pb/206Pb |
207Pb/235U |
206Pb/238U |
207Pb/235U |
206Pb/238U |
207Pb/206Pb |
|||||||||
|
3.3N HCl (1)* |
0.05737±4 |
0.0059±10 |
0.6298±14 |
0.0796±2 |
0.95 |
496±1 |
494±1 |
505.8±4 |
|||||||
|
3.3N HCl (3) |
0.057279±2 |
0.0056±4 |
0.6311±14 |
0.0799±2 |
0.98 |
497±1 |
496±1 |
502.3±2 |
|||||||
|
3.3N HCl (2) |
0.05734±3 |
0.0062±5 |
0.6317±14 |
0.0800±2 |
0.97 |
497±1 |
495±1 |
504.7±2 |
|||||||
|
2N HCl (3) |
0.057302±3 |
0.00584±8 |
0.6365±14 |
0.0806±2 |
0.97 |
500±1 |
499±1 |
503.2±2 |
|||||||
|
2N HCl (3) |
0.05734±2 |
0.0060±2 |
0.6349±14 |
0.0803±2 |
0.99 |
499±1 |
498±1 |
504.8±2 |
|||||||
* In parentheses is the number of garnet crystals used in the suspension.
** Calculated from 208Pb/206Pb isotopic ratios with consideration of age, values are given for the time of mineral crystallization.
*** Isotopic ratios corrected for mass fractionation and blank experiment.
**** Error correlation coefficients 207Pb/235U-206Pb/238U.
The figurative points of garnets form a compact subconcordant cluster on the diagram with concordia (Fig.4), through which the discordia is drawn (MSWD = 1.8). The lower intersection of discordia with concordia (16±320 Ma) is conventionally zero. The upper intersection has an age of 504.1±4.3 Ma, which can be considered as the time of garnet crystallization and, consequently, the formation of silicate-carbonate rocks within the Perovskitovaya mine.
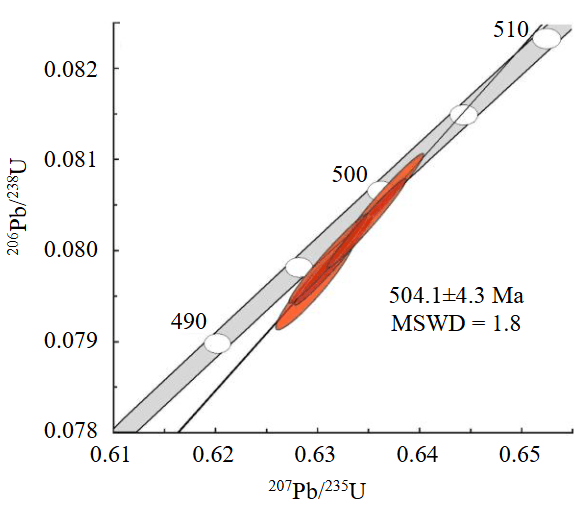
Fig.4. Concordia diagram of five analyses for garnets from silicate-carbonate rocks of the Perovskitovaya mine
Discussion
Mineralogical and geochemical features of titanite
An important feature of titanite considered in this work is its color – as a rule, white or pale beige with greenish tints. Apparently, such coloring of crystals is due to the low content of impurity elements (e.g., Fe, V, Cr, Mn, etc.). In addition, it is also necessary to note the low U content (less than 1 ppm for most of them) in the composition of titanite grains – with such U content it is difficult to conduct geochronological studies using the U-Pb method.
The results of statistical processing of data on titanite geochemistry by PCA method are shown in Fig.5. The total weight of two principal components is about 85 %. The diagram of loadings for the first and second principal component (Fig.5, a) demonstrates positive loadings for the first component V, Cr, and Sr, while for the other elements – negative. REE from La to Dy, as well as such highly charged elements as Hf and Ta have comparable modulus loads on the second principal component, with heavy REE (Er, Yb, Lu) and Y being characterized by the maximum positive load. On the diagram of values of the first and second principal components (Fig.5, b) figurative points of titanite from mineral aggregates of Praskovie-Evgenyevskaya and Nikolaje-Maksimilianovskaya mines form a single trend directed along the diagonal from the area of positive values of the first principal component to the area of positive values of the second principal component. This trend corresponds to the “evolution” of titanite compositions from enriched Sr, Cr, and V to enriched HREE and Y. The points of titanite composition from the Akhmatovskaya mine also form a trend with varying values of the first principal component, which corresponds to different levels of light REE and Th accumulation.
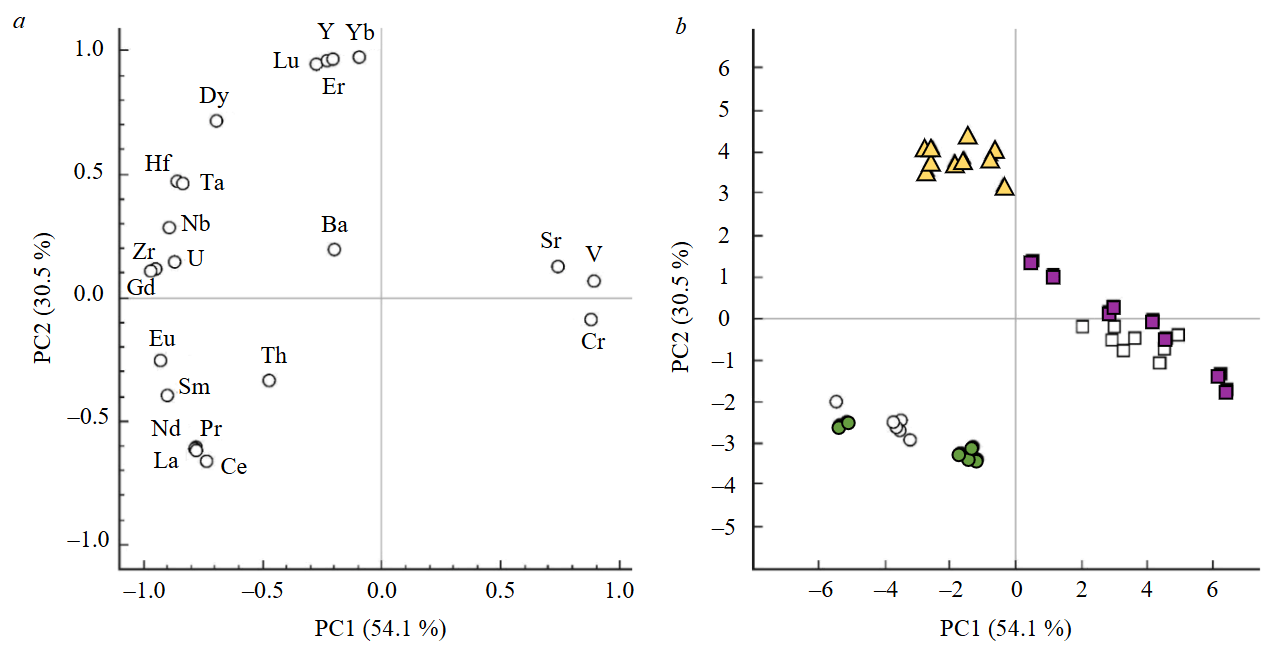
Fig.5. Visualization of element-impurities distribution data in titanite by PCA method in cocoordinates of the first and second principal components of the load diagram (a) and values of the principal component (b). The percentage in parentheses is the weight of the corresponding principal component (PC)
For legend, see Fig.2.
In general, by the first and second major components there was a separation of titanite composition points from different mineral aggregates into three clusters: in the Akhmatovskaya mine – enriched in LREE and Th; in the Nikolaje-Maksimilianovskaya mine – enriched in HREE, Hf, and Ta; in the Praskovie-Evgenyevskaya mine – enriched in V, Cr, and Sr. Relative enrichment of titanite in Sr, Cr, and V (Praskovie-Evgenyevskaya mine) probably reflects the factor of protolith – gabbro, rock containing these elements as plagioclases (Sr) and pyroxenes, as well as ore minerals – magnetite and/or ilmenite (Cr and V). For titanite from mineral aggregates of the Akhmatovskaya and Nikolaje-Maksimilianovskaya mines, the connection with gabbro is weak, so figurative points have negative values for the first major component. The second principal component can be interpreted as a factor of garnet presence in paragenesis with titanite. Being a concentrator of HREE and Y, garnet leads to depletion of coexisting titanite by these elements. For titanite from the mineral aggregate of the Nikolaje-Maksimilianovskaya mine, garnet is absent in the paragenesis, so this titanite shows maximum enrichment of heavy REE and Y. Titanite from mineral aggregates of the Akhmatovskaya mine is in contact with a significant amount of garnet, so it is depleted in heavy REE and Y.
The importance of mineralogical [36, 37] and geochemical [38-40] studies in solving the problems of petro- and ore-genesis was demonstrated earlier, and it was also established that the conditions of mineral genesis are reflected in the character of REE distribution in them [41-43]. The REE distribution spectra for titanite within each mineralogical copy correspond in principle to each other, which may indicate the commonality of the conditions of their formation within one object, even in spite of different mineral associations [44, 45]. The level of content and character of REE distribution in the studied samples are typical for titanite formed as a result of hydrothermal processes [46-48]. Titanite formed as a result of magmatic or metamorphic processes is characterized by other features of rare-element composition [5, 19, 49]. Despite this, the level of REE content and the manifestation of LREE differentiation relative to HREE may depend on both the composition of the parent rock and the crystallization sequence of titanite compared to other minerals [50]. It has been demonstrated that titanite is depleted in LREE when crystallizing together with or after LREE-concentrator minerals, such as epidote or apatite, and vice versa, HREE is depleted when crystallizing together with or after HREE-concentrator minerals, such as garnet or zircon [51].
Apparently, the peculiarities of REE distribution in the considered titanite are also related to the composition of paragenetic minerals (Fig.6). Thus, titanite from mineral aggregates of the Akhmatovskaya mine, which is in paragenesis with the HREE concentrator mineral – garnet, is enriched in LREE and depleted in HREE. The total REE content in titanite, which is in paragenesis with garnet and epidote, is lower than in titanite, which is in paragenesis with garnet only. This may be due to the accumulation of REE by epidote. Titanite from the mineral aggregate sampled in the Nikolaje-Maksimilianovskaya mine is depleted in LREE and enriched in HREE and is in paragenesis with epidote (a mineral-concentrator of LREE) and chlorite, which practically does not accumulate REE [52] and, therefore, does not influence the distribution of REE in titanite. In the case of titanite in paragenesis with “inert” chlorite in relation to REE and garnet (Praskovie-Evgenyevskaya mine), which is depleted in LREE and enriched in HREE, this can be explained by the influence of the composition of the protolith – gabbro. If titanite from mineral aggregates of the Akhmatovskaya and Nikolaje-Maksimilianovskaya mines was formed by hydrothermal process in rock’s cracks of contact-metasomatic origin (in skarn and in chlorite schist, respectively), then titanite from mineral aggregates of the Praskovie-Evgenyevskaya mine was formed in a fracture in an igneous rock (gabbro) and, probably, partially inherited the REE distribution of the host rock or rock-forming minerals for gabbro – for example, plagioclase or pyroxene.
In all titanite grains, Eu-anomaly is manifested to varying degrees, while a significant Ce-anomaly was established in single analyses of titanite from epidote-titanite-clinochlore association (Nikolaje-Maksimilianovskaya mine). Eu and Ce serve as indicators of redox conditions [46, 57, 58], but the excess of Eu and, consequently, the positive Eu-anomaly, in contact-metasomatic and hydrothermal processes may be due to the inheritance of Eu from plagioclases in the case of titanite development along them [47, 59, 60]. A noticeable positive Eu-anomaly in titanite from mineral aggregates of the Praskovie-Evgenyevskaya mine is probably inherited from plagioclases from the parent gabbros. If negative Ce-anomaly for titanite reflects reducing conditions, then weak positive Eu-anomaly at complete absence of Ce-anomaly can also be an indicator of reducing conditions.
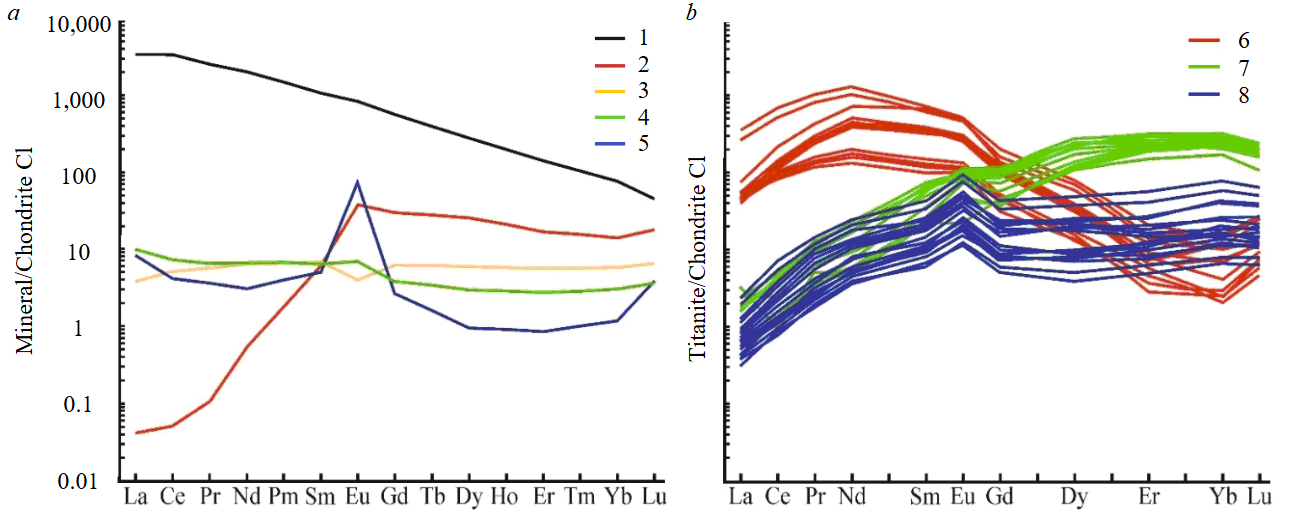
Fig.6. Distribution spectra of rare-earth elements for different minerals: а – 1-5 – based on averaged data from literature sources: 1 – magmatic titanite from rocks of alkaline-ultrabasic series of the Kola province [53], 2 – garnet from skarn mineral associations of the Akhmatovskaya mine [25], 3 – clinopyroxene from metabasite (Limpopo belt, South Africa) [54], 4 – epidote from skarns in the Petrovitsa deposit (Bulgaria) [55], 5 – plagioclase from various gabbroids [56]; б – 6-8 – of the considered titanite depending on paragenetic and “parent” minerals: 6 – with garnet (Akhmatovskaya mine), 7 – with epidote (Nikolaje-Maksimilianovskaya mine), 8 – by plagioclase and/or pyroxene (Praskovie-Evgenyevskaya mine)
Temperature of titanite formation
The results of calculations of the titanite formation temperature using Zr-thermometer [7] are given in Table 1-3. The temperature of titanite formation from mineral aggregates of the Akhmatovskaya mine is about 700 °C, which is probably the peak temperature and indicates the high-temperature regime of the supposed hydrothermal process. A similar temperature (about 700 °C) was obtained for titanite from mineral aggregate of the Nikolaje-Maksimilianovskaya mine. For titanite from mineral aggregates of the Praskovie-Evgenyevskaya mine, a lower temperature of about 580 °С was determined relative to titanite from other objects, which is probably associated with its formation at the regressive stage of the hydrothermal process. Such a variation in the temperature of titanite formation is essential for the hydrothermal process and requires an endogenous heat source, for example, intrusion of gabbroids or granitoids [61, 62]. Thus, the considered titanite-bearing parageneses could be formed as a result of hydrothermal process accompanying contact metasomatism.
U-Pb age of garnets
The age of garnets from silicate-carbonate rocks of the Perovskitovaya mine (504.1±4.3 Ma) is comparable to U-Pb age determinations (497-532 Ma) for perovskite from vein and vein bodies of chlorite-serpentine composition with druses of perovskite, magnetite, clinochlore, and calcite that cut silicate-carbonate rocks in the same mineral deposit [63]. However, these age estimates of about 500 Ma for garnet from silicate-carbonate rocks and perovskite from vein bodies differ significantly from the age of the host carbonate rocks of the Satka Formation, which is estimated to be 1,550±30 Ma [64, 65]. The age of the Kusa-Kopan intrusive complex, which penetrates the Satka Formation, is estimated in the range of 1,390-1,350 Ma [66]. The significant differences in the age of the intrusive and frame rocks from the age of silicate-carbonate rocks contradict the idea that the latter were formed as a result of contact metasomatosis synchronous with the intrusion of gabbroids or granitoids of the Kusa-Kopan complex, but do not exclude the influence of superimposed contact metasomatosis associated with late endogenous processes manifested on the western slope of the Southern Urals. There is a hypothesis that the silicate-carbonate rocks were formed as a result of Early Paleozoic low-grade metamorphism [67], but additional isotope-geochronologic studies are required to finally resolve these questions.
Conclusion
Mineralogical and geochemical studies of titanite from crystalline aggregates sampled in the mines of the Southern Urals demonstrate the dependence of titanite composition on the minerals with which it occurs in paragenesis and allow us to draw the following conclusions:
- The mineral associations containing titanite belong to four types: epidote-titanite-garnet (Akhmatovskaya mine); garnet-titanite-diopside (Akhmatovskaya mine); epidote-titanite-chlorite (Nikolaje-Maksimilianovskaya mine); chlorite-titanite-garnet (Praskovie-Evgenyevskaya mine). Directly paragenic minerals for titanite in them are: garnet and epidote; garnet; epidote and clinochlore; chlorite and garnet.
- According to the content of trace and rare-earth elements, titanite from different mineral aggregates is divided into three groups: Akhmatovskaya mine – enriched with LREE and Th; Nikolaje-Maksimilianovskaya mine – enriched with HREE, Hf, and Ta; Praskovie-Evgenyevskaya mine – enriched with V, Cr, and Sr.
- Peculiarities of REE distribution in titanite are related to the composition of paragenetic minerals. Titanite from mineral aggregates of the Akhmatovskaya mine, which is in paragenesis with HREE concentrator mineral – garnet, is enriched in LREE and depleted in HREE. Titanite from Nikolaje-Maksimilianovskaya mine is depleted in LREE and enriched in HREE and is in paragenesis with epidote (LREE concentrator mineral). Titanite from Praskovie-Evgenyevskaya mine is depleted in LREE and enriched in HREE due to the influence of the parent rock (gabbro) or its rock-forming minerals – plagioclase and pyroxene.
- The temperature of titanite formation from mineral aggregates of the Akhmatovskaya and Nikolaje-Maksimilianovskaya mines is about 700 °С. For titanite from mineral aggregates of Praskovie-Evgenyevskaya mine, the temperature of about 580 °С is lower than that of titanite from other objects. The established temperature values are essential for the hydrothermal process and are provided by the endogenous heat source (intrusion). Thus, the considered titanite-bearing paragenesis could be formed as a result of hydrothermal process accompanying contact metasomatosis.
As a result of U-Pb dating by ID-TIMS method, the age of garnets from silicate-carbonate rocks of the Perovskitovaya mine was determined to be 504.1±4.3 Ma. The obtained age estimates contradict the idea that silicate-carbonate rocks were formed as a result of contact metasomatosis synchronous with the intrusion of gabbroids or granitoids of the Kusa-Kopan complex (1,390-1,350 Ma), but do not exclude the influence of superimposed contact metasomatosis associated with late endogenous processes.
References
- Tiepolo M., Oberti R., Vannucci R. Trace-element incorporation in titanite: constraints from experimentally determined solid/liquid partition coefficients. Chemical Geology. 2002. Vol. 191. Iss. 1-3, p. 105-119. DOI: 10.1016/S0009-2541(02)00151-1
- Sharova O.I., Chudnenko K.V., Avchenko O.V. et al. Aluminum–Fluorine Sphene (Titanite) as an Indicator of Fluorine Fluid. Doklady Earth Sciences. 2012. Vol. 442, p. 126-129. DOI: 10.1134/S1028334X12010217
- Kowallis B.J., Christiansen E.H., Dorais M.J. et al. Variation of Fe, Al, and F Substitution in Titanite (Sphene). Geosciences. 2022. Vol. 12. Iss. 6. N 229. DOI: 10.3390/geosciences12060229
- Frost B.R., Chamberlain K.R., Schumacher J.C. Sphene (titanite): phase relations and role as a geochronometer. Chemical Geology. 2001. Vol. 172. Iss. 1-2, p. 131-148. DOI: 10.1016/S0009-2541(00)00240-0
- Kohn M.J. Titanite Petrochronology. Reviews in Mineralogy and Geochemistry. 2017. Vol. 83. N 1, p. 419-441. DOI: 10.2138/rmg.2017.83.13
- Ronghua Guo, Xiumian Hu, Eduardo Garzanti et al. How faithfully do the geochronological and geochemical signatures of detrital zircon, titanite, rutile and monazite record magmatic and metamorphic events? A case study from the Himalaya and Tibet. Earth-Science Reviews. 2020. Vol. 201. N 103082. DOI: 10.1016/j.earscirev.2020.103082
- Hayden L.A., Watson E.B., Wark D.A. A thermobarometer for sphene (titanite). Contributions to Mineralogy and Petrology. 2008. Vol. 155. Iss. 4, p. 529-540. DOI: 10.1007/s00410-007-0256-y
- Erdmann S., Rucheng Wang, Fangfang Huang et al. Titanite: A potential solidus barometer for granitic magma systems. Comptes Rendus Geoscience. 2019. Vol. 351. Iss. 8, p. 551-561. DOI: 10.1016/j.crte.2019.09.002
- Matthews T.J., Loader M.A., Wilkinson J.J. et al. The Strontian Intrusive Complex: Petrography, Thermobarometry and the Influence of Titanite on Residual Melt Chemistry. Journal of Petrology. 2023. Vol. 64. Iss. 8. N egad059. DOI: 10.1093/petrology/egad059
- Kirkland C.L., Yakymchuk C., Gardiner N.J. et al. Titanite petrochronology linked to phase equilibrium modelling constrains tectono-thermal events in the Akia Terrane, West Greenland. Chemical Geology. 2020. Vol. 536. N 119467. DOI: 10.1016/j.chemgeo.2020.119467
- Gros K., Słaby E., Birski Ł. et al. Geochemical evolution of a composite pluton: insight from major and trace element chemistry of titanite. Mineralogy and Petrology. 2020. Vol. 114. Iss. 5. p. 375-401. DOI: 10.1007/s00710-020-00715-x
- Lu Xiang, Jia Guo, Minghui Yin et al. Polygenetic titanites constraining the genesis of Neoproterozoic leucocratic-dyke-hosted U mineralization at the western margin of the Yangtze Block. Lithos. 2023. Vol. 438-439. N 107008. DOI: 10.1016/j.lithos.2022.107008
- Scibiorski E.A., Cawood P.A. Titanite as a petrogenetic indicator. Terra Nova. 2022. Vol. 34. Iss. 3, p. 177-183. DOI: 10.1111/ter.12574
- Marsh J.H., Smye A.J. U-Pb systematics and trace element characteristics in titanite from a high-pressure mafic granulite. Chemical Geology. 2017. Vol. 466, p. 403-416. DOI: 10.1016/j.chemgeo.2017.06.029
- Xiao-Dong Deng, Jian-Wei Li, Mei-Fu Zhou et al. In-situ LA-ICPMS trace elements and U-Pb analysis of titanite from the Mesozoic Ruanjiawan W–Cu–Mo skarn deposit, Daye district, China. Ore Geology Reviews. 2015. Vol. 65. Part 4, p. 990-1004. DOI: 10.1016/j.oregeorev.2014.08.011
- Xuan Dac Ngo, Xin-Fu Zhao, Thanh Hai Tran et al. Two episodes of REEs mineralization at the Sin Quyen IOCG deposit, NW Vietnam. Ore Geology Reviews. 2020. Vol. 125. N 103676. DOI: 10.1016/j.oregeorev.2020.103676
- Jia Dai Li, Xiao Feng Li, Rong Xiao. In situ LA-ICP-MS U-Pb geochronology and trace element analysis of hydrothermal titanite from the Jiepai W-Cu deposit, South China: Implications for W mineralization. The Canadian Mineralogist. 2020. Vol. 58. N 1, p. 45-69. DOI: 10.3749/canmin.1900027
- Yuzhou Feng, Yuanming Pan, Bing Xiao et al. Hydrothermal alteration of magmatic titanite: Implications for REE remobilization and the formation of ion-adsorption HREE deposits, South China. American Mineralogist. 2023. Vol. 108. Iss. 11, p. 2051-2064. DOI: 10.2138/am-2022-8644
- Garber J.M., Hacker B.R., Kylander-Clark A.R.C. et al. Controls on Trace Element Uptake in Metamorphic Titanite: Implications for Petrochronology. Journal of Petrology. 2017. Vol. 58. Iss. 6, p. 1031-1057. DOI: 10.1093/petrology/egx046
- Dolgov V.S., Sereda M.S., Kozlov A.V. Minerals of the Zlatoust Urals. Zlatoust: Foto-Mir, 2007, p. 208 (in Russian).
- Popov V.A. Mineralogical study of skarn and carbonatite of the Akhmat mine. Uralskii mineralogicheskii sbornik. 2010. N 17, p. 110-118 (in Russian).
- Melnikov M.P. Nikolaje-Maksimilianovskaya mineral mine near the Kusinsky plant in the Urals. Zapiski Imperatorskago S.-Peterburgskago mineralogicheskago obshchestva. 1885. N 20, p. 237-264 (in Russian).
- Popov V.A. To the mineralogy of the Praskovie-Evgenyevskaya mine in the Southern Urals. XVIII Vserossiiskaya nauchnaya konferentsiya “Uralskaya mineralogicheskaya shkola-2012, posvyashchennaya blagorodnym metallam (Au, Ag, Pt, Ir, Os, Pd, Rh, Ru)”: Sbornik statei studentov, aspirantov, nauchnykh sotrudnikov akademicheskikh institutov i prepodavatelei VUZov geologicheskogo profilya. Ekaterinburg: Institut geologii i geokhimii URO RAN, 2012, p. 134-139 (in Russian).
- Skublov S.G., Petrov D.A., Galankina O.L. et al. Th-Rich Zircon from a Pegmatite Vein Hosted in the Wiborg Rapakivi Granite Massif. Geosciences. 2023. Vol. 13. Iss. 12. N 362. DOI: 10.3390/geosciences13120362
- Stativko V.S., Skublov S.G., Smolenskiy V.V., Kuznetsov A.B. Trace and rare-earth elements in garnets from silicate-carbonate formations of the Kusa-Kopan complex (Southern Urals). Lithosphere. 2023. Vol. 23. N 2, p. 225-246 (in Russian). DOI: 10.24930/1681-9004-2023-23-2-225-246
- Krivovichev V.G., Gulbin Yu.L. Recommendations for mineral formula calculations from chemical analytical data. Geology of Ore Deposits. 2022. Vol. 151. N 1, p. 114-124 (in Russian). DOI: 10.31857/S0869605522010087
- McDonough W.F., Sun S.-s. The composition of the Earth. Chemical Geology. 1995. Vol. 120. Iss. 3-4. p. 223-253. DOI: 10.1016/0009-2541(94)00140-4
- Belonin M.D., Golubeva V.A., Skublov G.T. The factor analysis in geology. Moscow: Nedra, 1982, p. 269 (in Russian).
- Maćkiewicz A., Ratajczak W. Principal components analysis (PCA). Computers & Geosciences. 1993. Vol. 19. Iss. 3, p. 303-342. DOI: 10.1016/0098-3004(93)90090-R
- Abdi H., Williams L.J. Principal component analysis. WIREs Computational Statistics. 2010. Vol. 2. Iss. 4, p. 433-459. DOI: 10.1002/wics.101
- Olierook H.K.H., Taylor R.J.M., Erickson T.M. et al. Unravelling complex geologic histories using U-Pb and trace element systematics of titanite. Chemical Geology. 2019. Vol. 504, p. 105-122. DOI: 10.1016/j.chemgeo.2018.11.004
- Harrigan C.O., Trevino S.F., Schmitz M.D., Tikoff B. Determining the initiation of shear zone deformation using titanite petrochronology. Earth and Planetary Science Letters. 2024. Vol. 631. N 118620. DOI: 10.1016/j.epsl.2024.118620
- Skublov S.G., Gavrilchik A.K., Berezin A.V. Geochemistry of beryl varieties: comparative analysis and visualization of analytical data by principal component analysis (PCA) and t-distributed stochastic neighbor embedding (t-SNE). Journal of Mining Institute. 2022. Vol. 255, p. 455-469. DOI: 10.31897/PMI.2022.40
- Manhes G., Minster J.F., Allègre C.J. Comparative uranium-thorium-lead and rubidium-strontium study of the Saint Sèverin amphoterite: consequences for early solar system chronology. Earth and Planetary Science Letters. 1978. Vol. 39. Iss. 1, p. 14-24. DOI: 10.1016/0012-821X(78)90137-1
- Horwitz E.P., Deitz M.L., Chiarizia R. et al. Separation and preconcentration of uranium from acidic media by extraction chromatography. Analytica Chimica Acta. 1992. Vol. 266. Iss. 1, p. 25-37. DOI: 10.1016/0003-2670(92)85276-C
- Marin Yu.B. On Mineralogical Studies and the Use of Mineralogical Information in Solving Petro- and Ore Genesis Problems. Geology of Ore Deposits. 2021. Vol. 63. N 7, p. 625-633. DOI: 10.1134/S1075701521070059
- Gulbin Yu.L., Akbarpuran Khaiyati S.A., Sirotkin A.N. Mineral composition and thermobarometry of metamorphic rocks of Western Ny Friesland, Svalbard. Journal of Mining Institute. 2023. Vol. 263, p. 657-673.
- Levashova E.V., Skublov S.G., Hamdard N. et al. Geochemistry of Zircon from Pegmatite-bearing Leucogranites of the Laghman Complex, Nuristan Province, Afghanistan. Russian Journal of Earth Sciences. 2024. Vol. 24. Iss. 2. N ES2011 (in Russian). DOI: 10.2205/2024ES000916
- Levashova E.V., Mamykina M.E., Skublov S.G. et al. Geochemistry (TE, REE, Oxygen) of Zircon from Leucogranites of the Belokurikhinsky Massif, Gorny Altai, as Indicator of Formation Conditions. Geochemistry International. 2023. Vol. 61. N 13, p. 1323-1339. DOI: 10.1134/S001670292311006X
- Skublov S.G., Hamdard N., Ivanov M.A., Stativko V.S. Trace element zoning of colorless beryl from spodumene pegmatites of Pashki deposit (Nuristan province, Afghanistan). Frontiers in Earth Science. 2024. Vol. 12, p. 1432222. DOI: 10.3389/feart.2024.1432222
- Dubinin A.V. Rare earth element geochemistry in the ocean. Moscow: Nauka, 2006, p. 360 (in Russian).
- Skublov S.G., Levashova E.V., Mamykina M.E. et al. The polyphase Belokurikhinsky granite massif, Gorny Altai: isotope-geochemical study of zircon. Journal of Mining Institute. 2024. Vol. 268, p. 552-575.
- Skublov S.G. Geochemistry of rare-earth elements in rock-forming metamorphic minerals. SPb: Nauka, 2005. 147 p.
- Akbarpuran Haiyati S.A., Gulbin Yu.L., Sirotkin A.N., Gembitskaya I.M. Compositional Evolution of REE- and Ti-Bearing Accessory Minerals in Metamorphic Schists of Atomfjella Series, Western Ny Friesland, Svalbard and Its Petrogenetic Significance. Geology of Ore Deposits. 2021. Vol. 63. N 7, p. 634-653. DOI: 10.1134/S1075701521070047
- Burlakova A.A., Smolenskii V.V., Vilkin G.S., Konstantinova N.P. Mineralogical and geochemical features of low-temperature hydrothermal formations of Ashadze-2 and Petersburg ore fields (SAH). The 10th International Conference “Minerals of the Ocean”. Abstract Book, 20-22 June 2023, Saint Petersburg, Russia. St. Petersburg: VNIIOkeangeologia, 2023, p. 77-80 (in Russian).
- Horie K., Hidaka H., Gauthier-Lafaye F. Elemental distribution in apatite, titanite and zircon during hydrothermal alteration: Durability of immobilization mineral phases for actinides. Physics and Chemistry of the Earth, Parts A/B/C. 2008. Vol. 33. Iss. 14-16, p. 962-968. DOI: 10.1016/j.pce.2008.05.008
- Jian-Wei Li, Xiao-Dong Deng, Mei-Fu Zhou et al. Laser ablation ICP-MS titanite U–Th–Pb dating of hydrothermal ore deposits: A case study of the Tonglushan Cu–Fe–Au skarn deposit, SE Hubei Province, China. Chemical Geology. 2010. Vol. 270. Iss. 1-4, p. 56-67. DOI: 10.1016/j.chemgeo.2009.11.005
- Paoli G., Dini A., Petrelli M., Rocchi S. HFSE-REE Transfer Mechanisms During Metasomatism of a Late Miocene Peraluminous Granite Intruding a Carbonate Host (Campiglia Marittima, Tuscany). Minerals. 2019. Vol. 9. Iss. 11. N 682. DOI: 10.3390/min9110682
- Prowatke S., Klemme S. Effect of melt composition on the partitioning of trace elements between titanite and silicate melt. Geochimica et Cosmochimica Acta. 2005. Vol. 69. Iss. 3, p. 695-709. DOI: 10.1016/j.gca.2004.06.037
- Bruand E., Storey C., Fowler M. Accessory Mineral Chemistry of High Ba–Sr Granites from Northern Scotland: Constraints on Petrogenesis and Records of Whole–rock Signature. Journal of Petrology. 2014. Vol. 55. Iss. 8, p. 1619-1651. DOI: 10.1093/petrology/egu037
- Papapavlou K., Darling J.R., Storey C.D. et al. Dating shear zones with plastically deformed titanite: New insights into the orogenic evolution of the Sudbury impact structure (Ontario, Canada). Precambrian Research. 2017. Vol. 291, p. 220-235. DOI: 10.1016/j.precamres.2017.01.007
- Wei Tan, Qigui Mao, Mingjie Yu et al. Mineralization of the Tuwu Porphyry Cu Deposit in Eastern Tianshan, NW China: Insights From In Situ Trace Elements of Chlorite and Pyrite. Frontiers in Earth Science. 2021. Vol. 9. N 648177. DOI: 10.3389/feart.2021.648177
- Arzamastsev A.A., Arzamastseva L.V. Geochemical Indicators of the Evolution of the Ultrabasic-Alkaline Series of Paleozoic Massifs of the Fennoscandian Shield. Petrology. 2013. Vol. 21. N 3, p. 249-279. DOI: 10.1134/S0869591113020021
- Buick I.S., Hermann J., Maas R., Gibson R.L. The timing of sub-solidus hydrothermal alteration in the Central Zone, Limpopo Belt (South Africa): Constraints from titanite U–Pb geochronology and REE partitioning. Lithos. 2007. Vol. 98. Iss. 1-4, p. 97-117. DOI: 10.1016/j.lithos.2007.02.002
- Hantsche A.L., Kouzmanov K., Milenkov G. et al. Metasomatism and cyclic skarn growth along lithological contacts: Physical and geochemical evidence from a distal Pb–Zn skarn. Lithos. 2021. Vol. 400-401. N 106408. DOI: 10.1016/j.lithos.2021.106408
- Lesnov F.P. Concentration of rare-earth elements in plagioclases from rocks of different composition and genesis. Petrology of magmatic and metamorphic complexes. Reports from science meeting, 29-30 March 2000, Tomsk, Russia. Tomsk: CSTI, 2000, p. 38-42 (in Russian).
- Li-Chuan Pan, Rui-Zhong Hu, Xian-Wu Bi et al. Titanite major and trace element compositions as petrogenetic and metallogenic indicators of Mo ore deposits: Examples from four granite plutons in the southern Yidun arc, SW China. American Mineralogist. 2018. Vol. 103. Iss. 9, p. 1417-1434. DOI: 10.2138/am-2018-6224
- Shiwei Song, Jingwen Mao, Guiqing Xie et al. In situ LA-ICP-MS U-Pb geochronology and trace element analysis of hydrothermal titanite from the giant Zhuxi W (Cu) skarn deposit, South China. Mineralium Deposita. 2019. Vol. 54. Iss. 4, p. 569-590. DOI: 10.1007/s00126-018-0831-3
- Ismail R., Ciobanu C.L., Cook N.J. et al. Rare earths and other trace elements in minerals from skarn assemblages, Hillside iron oxide–copper–gold deposit, Yorke Peninsula, South Australia. Lithos. 2014. Vol. 184-187, p. 456-477. DOI: 10.1016/j.lithos.2013.07.023
- Leiluo Xu, Xianwu Bi, Ruizhong Hu et al. LA-ICP-MS mineral chemistry of titanite and the geological implications for exploration of porphyry Cu deposits in the Jinshajiang – Red River alkaline igneous belt, SW China. Mineralogy and Petrology. 2015. Vol. 109. Iss. 2, p. 181-200. DOI: 10.1007/s00710-014-0359-x
- Averev V.V. Hydrothermal process in volcanic areas and its relation to magmatic activity. Sovremennyi vulkanizm. Trudy vtorogo Vsesoyuznogo vulkanologicheskogo soveshchaniya, 3-17 sentyabrya 1964 g. Moscow: Nauka, 1966. Vol. 1, p. 118-128 (in Russian).
- Kucherenko I.V. Theories, hypotheses of hydrothermal rock-ore-formation and reality: facts and arguments. Bulletin of the Tomsk Polytechnic University. Geo Assets Engineering. 2015. Vol. 326. N 10, p. 99-122 (in Russian).
- Stepanov S.Yu., Puchkov V.N., Palamarchuk R.S. et al. The first evidence of Paleozoic endogenous activity on the Western slope of the Southern Urals. Doklady Rossiiskoi akademii nauk. Nauki o Zemle. 2020. Vol. 493. N 1, p. 21-26. DOI: 10.31857/S2686739720070208
- Kuznetsov A.B., Ovchinnikova G.V., Semikhatov M.A. et al. The Sr Isotopic Characterization and Pb-Pb Age of Carbonate Rocks from the Satka Formation, the Lower Riphean Burzyan Group of the Southern Urals. Stratigraphy and Geological Correlation. 2008. Vol. 16. N 2, p. 120-137. DOI: 10.1134/S0869593808020020
- Semikhatov M.A., Kuznetsov A.B., Maslov A.V. et al. Stratotype of the Lower Riphean, the Burzyan Group of the Southern Urals: Lithostratigraphy, Paleontology, Geochronology, Sr- and C-isotopic Characteristics of Its Carbonate Rocks. Stratigraphy and Geological Correlation. 2009. Vol. 17. N 6, p. 574-601. DOI: 10.1134/S0869593809060021
- Kholodnov V.V., Fershtater G.B., Ronkin Y.L. et al. Sm–Nd and Rb–Sr Ages of Gabbroids, Granitoids, and Titanomagnetite Ores from Layered Intrusions of the Kusa–Kopan Complex (South Urals). Doklady Earth Sciences. 2010. Vol. 432. Part 2, p. 732-736. DOI: 10.1134/S1028334X10060048
- Gekimyants V.M. The mineralogy of titanium and zirconium in skarns, roddingites and roddingite-like formations in the Western Urals: Avtoref. dis. ... kand. geol.-mineral. nauk. Moscow, 2000, p. 21 (in Russian).
