Theoretical and experimental substantiation of using Fe0-C redox system for nitrate ion removal from quarry waters
- 1 — Postgraduate Student Perm National Research Polytechnic University ▪ Orcid
- 2 — Ph.D., Dr.Sci. Professor Perm National Research Polytechnic University ▪ Orcid
Abstract
Quarry wastewater from open-pit iron ore mining enterprises is a source of contamination of surface water bodies and groundwater with chemical compounds used during development, including the products of decomposition and incomplete consumption of ammonium nitrate during blasting operations in mines – nitrate, nitrite, and ammonium nitrogen. Such characteristics of mining wastewater as high tonnage, organic matter deficiency, and sparse microbiome must be considered when selecting neutralization methods. Biological and physicochemical methods are used to treat wastewater contaminated with nitrogen compounds. Some methods are economically infeasible due to the significant volumes of wastewater generated. An important task is to find an economically viable and highly effective method for treating quarry water from nitrogen compounds. The article presents the results of theoretical and experimental studies of the possibility of using a permeable geochemical barrier based on a redox system consisting of iron scrap and carbon-containing material (screenings from the production of birch activated charcoal) for treating quarry waters from nitrate ions. Thermodynamic analysis allowed us to determine the chemistry of nitrate ion reduction by the Fe0-C redox system in a neutral and slightly alkaline medium typical of quarry waters. The study of the kinetic patterns of nitrate ion reduction showed that the process rate is described by a first-order equation. It was found that the rate constant for nitrate ion reduction increases with reaction mixture temperature rise: at 278 K – 0.0365 min–1, 283 K – 0.0416 min–1, 288 K – 0.0809 min–1, 293 K – 0.0901 min–1. The data obtained will allow substantiating the choice of the reactive barrier or reactor design for the treatment. Experimental studies on the treatment of real and model quarry waters in a laboratory setup simulating a geochemical barrier proved the high efficiency of nitrate ion reduction (more than 97 %). The treated water meets the requirements for water discharge into fishery water bodies.
Introduction
Poor quality of water resources in areas adjacent to mining sites is a global environmental problem [1, 2]. Inorganic nitrogen compounds are included in the list of pollutants, the content of which in quarry waters of mining enterprises exceeds sanitary standards [3], and their leaching from emulsions and suspensions used in drilling and blasting operations is considered the main reason for their entry into wastewater [4-6].
High content of inorganic nitrogen in water bodies causes eutrophication, rapid accumulation of toxic substances, and death of aquatic organisms [7]. Water supply sources in areas affected by mining enterprises, in particular groundwater, may be partially suitable or unsuitable for irrigation and domestic purposes [8, 9]. Health risk assessment studies show that drinking water with nitrate ion content exceeding sanitary standards poses high health risks [10], such as methemoglobinemia and thyroid hormone dysfunction [11]. Children are particularly vulnerable to the harmful effects of nitrate ions [12, 13].
It is proposed to reduce the negative impact of mines on hydrological bodies by biological and physicochemical methods. Biological methods for removing inorganic nitrogen compounds include microbial denitrification methods [14], as well as the use of constructed wetlands [15]. The undoubted advantages of quarry water phytoremediation methods are low operating costs and functioning based on biotic mechanisms combining the assimilation of inorganic nitrogen by higher aquatic vegetation [16] and nitrification-denitrification mechanisms by microorganisms [17]. However, nitrogen-containing wastewater treatment efficiency is affected by seasonal temperature fluctuations [18-20], and in extreme climatic conditions, the use of phytoremediation may require additional measures to maintain the performance of treatment facilities [21, 22]. Therefore, researchers classify wastewater treatment technology using constructed wetlands as extensive technologies [23]. Studies indicate a significant impact of carbon deficiency as an electron donor on the wastewater denitrification efficiency [24, 25].
Physicochemical methods, such as membrane technologies for treating mining wastewater from nitrogen compounds, demonstrate high efficiency [26, 27]. However, the application of this method to large-tonnage quarry wastewater from mining enterprises requires significant capital and operating costs [28].
Currently, permeable reactive barrier technology is used to treat wastewater containing oxidizing ions [29]. The technology is implemented as a subsurface material capable of entering into chemical reactions with pollutants, transforming them into less toxic forms [30]. Zero-valent iron, exhibiting reducing properties, is currently the main material for constructing permeable reactive barriers [31]. Its use allows achieving high results in treating areas from a wide range of oxidizing pollutants [32, 33]. However, information on the mechanisms of nitrate ion transformation when using reactive materials is contradictory. Analysis of scientific and technical information showed that the reduction reaction of nitrate ions with zero-valent iron is proposed to be carried out in an acidic medium [34]. In these conditions, the main product of nitrate ion reduction is ammonium ions [35], the content of which is also strictly regulated. The pH value of quarry waters ranges from 6 to 8, and conducting the process in an acidic medium would require significant expenditure on reagents.
Some researchers point to the possibility of gaseous nitrogen formation as a result of the interaction between nitrate ions and a material containing Fe0 and Fe2+ [36].
Previous studies [37] showed the high efficiency of a reactive system consisting of zero-valent iron and waste from activated carbon production for the treatment of quarry wastewater from nitrate ions. During the electrochemical processes, a number of reducing agents are formed that interact with nitrate ions. Analysis of the composition of treated quarry waters showed an insignificant content of ammonium ions and nitrite ions, suggesting that nitrogen is the main product of nitrate ion reduction.
It was found that the processes occurring in the Fe0-C redox system during the treatment of neutral and slightly alkaline quarry waters from nitrate ions, as well as their rate, differ significantly from the data presented in studies on the application of a permeable reactive barrier containing zero-valent iron for treating contaminated wastewater with low pH values. In this regard, in order to determine the mechanism of nitrate ion reduction by the Fe0-C galvanic couple in solutions close to neutral and slightly alkaline, there was a need for a more detailed study of the processes occurring in the reaction medium.
The aim of this work is to provide theoretical and experimental substantiation for the use of a redox system consisting of a mixture of iron turnings and carbon-containing material for the treatment of quarry waters from nitrate ions.
Methods
The studies were conducted both on real quarry wastewater and on solutions prepared in the laboratory, simulating the chemical composition of quarry water. The chemical composition of quarry waters from the mining enterprise is presented in Table 1 (based on long-term monitoring studies).
Table 1
Chemical composition of quarry water from a mining enterprise
|
Component |
MPCfish*, mg/dm3 |
Concentration, mg/dm3 |
|
|
Maximum values |
Average values |
||
|
рН |
Background values |
8.1 |
7.6 |
|
Ammonium ion |
0.5 (0.4 mgN/dm3) |
79.8 |
26.8 |
|
Manganese |
0.01 |
1.02 |
0.46 |
|
Nitrite ion |
0.08 (0.02 mgN/dm3) |
4.38 |
1.71 |
|
Nitrate ion |
40 (9 mgN/dm3) |
599.4 |
230.8 |
|
Sulphate ion |
100 |
643.0 |
378.1 |
|
Chloride ion |
300.0 |
188.2 |
66.4 |
|
Total iron |
0.1 |
1.8 |
0.5 |
|
Dry residue |
1000 |
1998 |
1919 |
* MPCfish – standards for maximum permissible concentrations of harmful substances in fishery water bodies, approved by Order of the Ministry of Agriculture of the Russian Federation dated 13 December 2016 N 552.
To obtain model solutions, chemically pure reagents and tap water were used.
In the study of quarry water treatment from nitrate ions using the Fe0-C redox system, iron-containing waste from metalworking production was applied – iron scrap (turnings) with a particle size of 3.5-4 mm and waste from the birch activated charcoal production (fraction 3-4 mm). Iron turnings were purified from contaminants and washed with distilled water. To activate iron corrosion, the galvanic couple was pre-treated with a 0.1 N hydrochloric acid solution.
The experiments were conducted both in static and dynamic modes.
In static mode, kinetic patterns of the nitrate ion reduction were investigated in reaction vessels. Model solutions of quarry waters with a volume of 1 dm3 and pH 7.0 were treated with the Fe0-C galvanic couple at temperatures of 278, 283, 288, and 293 K under constant stirring. The galvanic couple mass was 5 g, with a mass ratio of Fe0-C 2:1. To plot the kinetic curve, the nitrate ion content in samples was determined at specified time intervals, from 10 to 90 min. The concentration of nitrate ions in the solutions was 88.1±18.0 mgN/dm3.
In dynamic mode, the efficiency of quarry water treatment was investigated using a laboratory setup simulating the operation of a geochemical barrier. The setup consisted of a 0.5 dm3 model filter containing a mixture of iron scrap, activated carbon screenings, and sand (Fe0-C loading volume was 0.43 dm3), with a layer height of 14 cm. The Fe0-C mass ratio was 2:1. Quarry or model water with an initial nitrate ion concentration ranging from 81.8±16.0 to 137.2±27.0 mgN/dm3, pH 7.0-7.6, and temperature T = 293 K passed through the filter at a constant rate. The filtrate was collected in portions of 1.5-2 dm3, in which nitrate, nitrite, and ammonium ions were estimated.
Mass concentration of nitrate ions in the original and treated samples was determined photometrically with salicylic acid (PND F 14.1:2:4.4-95). The ammonium ion content was determined according to the procedure for performing quantitative chemical analysis with Nessler reagent (PND F 14.1:2:3.1-95). The nitrite ion content was controlled photometrically with Griess reagent (PND F 14.1:2:3:4.3-95). Photometric determinations were performed using an Ecoview-1200 spectrophotometer.
The operation of the laboratory setup was accompanied by the formation of a fine-crystalline precipitate, the composition of which was analysed by X-ray phase diffraction using a Shimadzu XRD-7000 X-ray diffractometer. X-ray diffraction patterns were processed in XRD 5.21 software.
Discussion
The electrochemical method for treating quarry waters from nitrogen compounds (nitrate and nitrite ions) is based on the interaction between the components of a galvanic couple (redox system) – materials with different electrochemical potential. Zero-valent iron, with a standard electrode potential E0 of −0.44 V, acts as an anode in the system, undergoes oxidation, and is subjected to hydrolysis according to the reaction equations:
Carbon, with a standard electrode potential of +0.475 V, performs the functions of cathode regions in the studied system. At the cathode regions, hydrogen reduction from water or oxygen dissolved in water proceeds in accordance with the following reactions:
To theoretically substantiate the most probable reactions of nitrate ion reduction in the Fe0-C redox system, a thermodynamic analysis of the processes was performed. The values of standard Gibbs free energy ∆G298 and equilibrium constants Ke of redox reactions were determined. These parameters serve as criteria for the direction and extent of spontaneous processes.
The change in standard Gibbs energy for a redox reaction is determined according to the formula:
where n is the number of electrons transferred in the redox reaction; F is the Faraday constant; E0 is the value of the electromotive force (EMF) of the reaction, defined as the difference between the standard electrode potentials of the oxidizing and reducing agents.
The standard Gibbs energy is the basis for estimating the reaction equilibrium constants at any temperature according to the formula ΔG0T=-RTlnKe:
The results of thermodynamic analysis of possible nitrate ion reduction reactions by the Fe0-C galvanic couple are presented in Table 2.
Analysis of the obtained data showed that in the Fe0-C redox system, nitrate ion reduction is possible with the formation of several products, the formation of which depends on the pH of the medium and the nature of the reducing agent (Fe0, Fe2+, Fe(OH)2, H2). The following reduction processes may proceed:
- nitrate ions to nitrite ions in the pH range from neutral (reactions 1-6) to alkaline (reaction 8);
- nitrate ions to ammonium ions in neutral medium (reactions 9, 11);
- nitrate ions to nitrogen oxide (II) in neutral (reactions 7, 10) and acidic (reaction 13) media;
- nitrate ions to gaseous nitrogen in acidic (reactions 12, 15, 16, 18) and neutral (reactions 14, 17, 19) media.
Table 2
Thermodynamic analysis of nitrate ion reduction reactions by Fe0-C galvanic couple (standard conditions)
|
Item N |
Reaction equation |
ΔG298, J |
Ke |
|
1 |
|
−81,832 |
1014 |
|
2 |
|
−86,850 |
1015 |
|
3 |
|
−90,845 |
1016 |
|
4 |
|
−91,038 |
1016 |
|
5 |
|
−110,010 |
1019 |
|
6 |
|
−118,058 |
1021 |
|
7 |
|
−158,646 |
1028 |
|
8 |
|
−171,191 |
1030 |
|
9 |
|
−263,020 |
1046 |
|
10 |
|
−331,217 |
1058 |
|
11 |
|
−339,680 |
1060 |
|
12 |
|
−458,375 |
1080 |
|
13 |
|
−810,600 |
10142 |
|
14 |
|
−1,380,046 |
10242 |
|
15 |
|
−1,596,110 |
10280 |
|
16 |
|
−1,621,200 |
10285 |
|
17 |
|
−1,697,531 |
10298 |
|
18 |
|
−1,777,240 |
10312 |
|
19 |
|
−1,853,572 |
10326 |
As can be seen from the presented data, the products of nitrate ion reduction depend on the pH of the medium. At the same time, in a wide pH range, reactions that proceed with the formation of gaseous products, including nitrogen, are thermodynamically most probable.
The formation of stable forms of iron and nitrogen compounds can be graphically represented as a diagram showing the dependence between the medium’s pH and the redox potential value Eh of the system (Pourbaix diagrams). Fig.1 shows the Eh-pH diagram for the Fe-N-H2O system, constructed based on the reference data on the electrode potential values and pH of hydration (the studied pH range is highlighted by a grey rectangle).
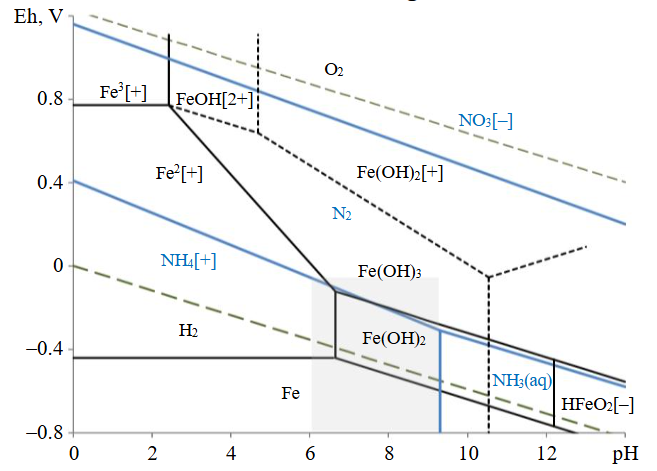
Fig.1. Eh-pH diagram for the Fe-N-H2O system, standard conditions (hydrated form of oxides)
Iron oxidation in the pH range of 6-9 lead to the formation of Fe2+, Fe(OH)2+ ions, as well as Fe (II) and (III) hydroxides. In this case, the products of nitrate ion reduction are nitrogen and ammonium ions, which is confirmed by the results of thermodynamic estimations (Table 2).
In article [38] studied the co-extraction of nitrate and Fe (III) ions using a material based on steelmaking slag. Referencing the research by D.Lewis, O.E.Zvyagintsev and Yu.S.Lopatto, T.G.Spiro, S.E.Allerton, and J.Renner, the pos-sibility of forming a series of Fe (III) polyhydroxy complexes with chain and spherical structures was demonstrated. It was also indicated that polycondensation of binuclear aquahyd-roxoiron (III) complexes is accompanied by the binding of nitrate ions into structures of the [Fe4O3(OH)5]NO3 type.
The economic feasibility of using the Fe0-C redox system for treating large-tonnage quarry wastewater from nitrate ions is determined by the rate of the electrochemical reaction.
In this regard, the kinetic patterns of nitrate ion reduction by the Fe0-C galvanic couple were studied. The study used model solutions of quarry waters with a nitrate ion concentration of 88.1±18.0 mgN/dm3. The dependence of the change in nitrate ion concentration on the contact time was determined at temperatures of 278, 283, 288 and 293 K (5-20 °C).
To determine the reaction order, kinetic curves were plotted on the lnC0/С of nitrate ions versus time axes. It was found that they can be described with a high degree of approximation by a first-order equation:
where ν is the reaction rate; t is the contact time; k is the rate constant of the chemical reaction.
Based on the obtained dependencies, the values of the rate constants for the electrochemical reduction of nitrate ions by the Fe0-C galvanic couple were determined at temperatures of 278, 283, 288, and 293 K. The results are shown in Fig.2.
The rate constant of the electrochemical reduction of nitrate ions increases with process temperature rise: k = 0.0365 min–1 at T = 278 K, k = 0.0416 min–1 at 283 K, k = 0.0809 min–1 at 288 K, k = 0.0901 min–1 at 293 K.
Comparison of the obtained data with the known scientific and technical information showed that the rate of NO3– ion reduction by the Fe0-C redox system is higher than in studies using macrosized particles of zero-valent iron. In those studies, at an initial nitrate ion concentration of 50 mg/dm3 and an iron dose of 100 g/dm3 at pH 2.5, the constant k = 0.0052 min–1 [39]. The rate constant in the studied Fe0-C system is higher compared to the data on nitrate ion reduction using powdered iron. In the latter case, at pH 2.5 and 6.7 and Fe0 dose of 10 g/dm3, k is equal to 0.016 and 0.0043 min–1, respectively.
Based on the results of kinetic analysis using the Arrhenius equation, the activation energy EА value for the nitrate ion reduction reaction by the Fe0-C redox system was estimated:
where R is the universal gas constant; k1 is the reaction rate constant at temperature T1; k2 is the reaction rate constant at temperature T2,
It was found that the activation energy EA value for the nitrate ion reduction reaction by the Fe0-C redox system is equal to 53 kJ/mol and is in the kinetic region. The chemical reaction is the rate-limiting stage in nitrate ion reduction by the studied redox system.
The results of kinetic analysis can be used to estimate the contact time between wastewater and the Fe0-C galvanic couple required to reduce the concentration of nitrate ions in quarry water to the maximum permissible concentration (MPC). Furthermore, these results will allow for the substantiation of the choice of the reactive barrier or reactor design for the treatment process.
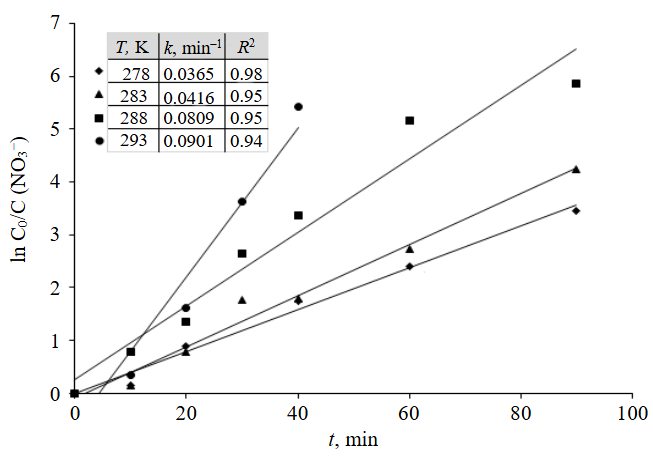
Fig.2. Curves showing the dependence of lnC0/С of nitrate ions on reaction time С0(NO3–) = 88.1±18.0 mgN/dm3; СFe-C = 5 g/dm3; pH 7.0; stirring rate 250 rpm; R2 – approximation reliability value
In the practice of treating large-tonnage wastewater, it is most expedient to conduct the process in a dynamic mode.
To experimentally substantiate the use of the Fe0-C redox system for treating quarry waters from nitrate ions in a permeable redox barrier, studies were conducted on a model setup using both real and model wastewater with a pH of 7.0-7.6 and a nitrate ion concentration ranging from 81.8±16.0 to 137.2±27.0 mgN/dm3. The wastewater feed rate to the setup corresponded to the contact time required for the electrochemical reaction to proceed, in accordance with the results of the kinetic analysis. The research results are presented in Table 3.
Table 3
Results of studies of nitrate ion reduction by Fe0-C galvanic couple in dynamic mode (T = 293 K)
|
Volume of solution passed in series, column volumes |
Total volume of solution passed, column volumes |
Concentration of nitrate ions before treatment, mgN/dm3 |
Concentration of pollutants after redox system, mgN/dm3 |
Degree of treatment from nitrate ions, % |
||
|
NO3– |
NO2– |
NH4+ |
||||
|
77 |
77 |
81.8±16.0 |
0.4±0.1 |
0.12±0.02 |
8.9±1.9 |
99.5 |
|
25 |
102 |
125.9±25.0 |
2.7±0.5 |
0.19±0.03 |
13.2±2.8 |
97.9 |
|
127 |
229 |
105.4±21.0 |
1.2±0.2 |
b.d.l.* |
8.4±1.8 |
98.9 |
|
95 |
324 |
95.4±19.0 |
2.6±0.5 |
0.24±0.03 |
2.8±0.6 |
97.3 |
|
53 |
377 |
137.2±27.0 |
2.9±0.6 |
0.50±0.07 |
1.8±0.4 |
97.9 |
*b.d.l. – below detection limit.
The obtained data indicate that when more than 377 column volumes passed through the system, which corresponds to more than 160 dm3, the treatment efficiency for nitrate ions was at least 97 % and the NO3– content in the filtrate was significantly lower than the MPCfish (40 mg/dm3 or 9 mgN/dm3). A visual assessment of the redox system’s state showed that the iron turnings were oxidized by less than one third.
After the solutions passed through the setup, an increase in pH value to 7.9-8.2 was observed. This is due to oxygen and hydrogen depolarization proceeding at the cathode regions of the galvanic couple, accompanied by the accumulation of hydroxyl groups in the near-cathode space.
The material balance estimation of quarry water treatment for nitrogen shows that the major part of nitrate ions is reduced to gaseous compounds, which is consistent with thermodynamic estimations as well as data presented in the literature [36, 40].
To substantiate the mechanism of the processes occurring in the studied redox system, an analysis of the solid phases formed during iron oxidation was conducted. The results of the X-ray phase analysis of the forming solid phase sample are presented in Fig.3.
Goethite α-FeOOH (interplanar distances, Å: 4.18095 (21.2°); 2.69207 (33.23°); 2.44935 (36.7°) and magnetite Fe3O4 (interplanar distances, Å: 2.95759 (30.2°); 2.5239 (35.5°); 2.09152 (43.2°); 1.47991 (62.7°) were identified in the sample.
The data obtained are consistent with the results of thermodynamic analysis of the processes occurring in the system under consideration and indicate that the reactions proceed in a weakly alkaline medium. The diffractogram (Fig.3) also shows peaks of calcite CaCO3 (interplanar distances, Å: 3.02751 (29.5°); 1.90917 (47.6°); 1.87149 (48.6°), which can form during the treatment of quarry waters containing hydrocarbonate ions.
The data obtained are generally consistent with studies in the field of geochemical modelling of permeable reactive barriers [41, 42].
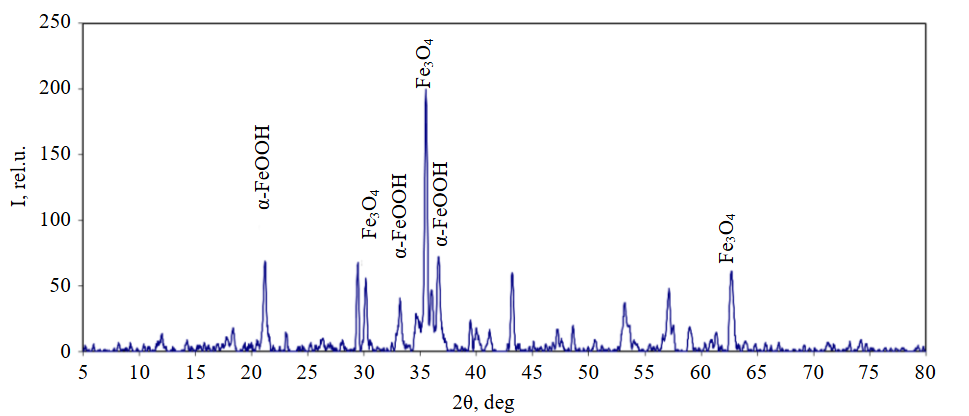
Fig.3. Diffraction pattern of the solid phase sample formed during nitrate ion reduction by a Fe0-C galvanic couple
Conclusion
A review of wastewater treatment practices at mining enterprises showed that finding an effective and economically feasible technology for treating large-tonnage quarry wastewater from nitrogen compounds is currently an urgent task.
The presented study shows the possibility of treating neutral and slightly alkaline quarry waters from nitrate ions using a Fe0-C galvanic couple. Based on the thermodynamic analysis of nitrate ion reduction by the Fe0-C redox system, the most probable reactions and main reduction products were determined. It was shown that in a neutral medium, reactions with the formation of ammonium ions and gaseous nitrogen are possible. Reactions proceeding with the formation of gaseous nitrogen are thermodynamically the most probable.
Experimental data show high efficiency of applying a redox system composed of iron scrap and carbon material for reducing nitrate ion content in quarry wastewater. The efficiency is ensured by the presence of several strong reducing agents in the redox system – zero-valent iron, Fe (II) ions, hydrogen formed at the cathode regions of the galvanic couple, and Fe (II) hydroxide.
The precipitate formed during the redox system operation is a mixture of thermodynamically stable phases of oxygen-containing iron compounds – goethite and magnetite.
The study of kinetic patterns of nitrate ion reduction by the Fe0-C redox system allowed determining the order and rate constant of the electrochemical reaction at temperatures of 278, 283, 288, and 293 K. The process rate is described by a first-order equation, with the rate constant k increasing as the reaction mixture temperature rises: k = 0.0365 min–1 at T = 278 K, k = 0.0416 min–1 at 283 K, k = 0.0809 min–1 at 288 K, k = 0.0901 min–1 at 293 K. The activation energy EA value of the nitrate ion reduction by the Fe0-C redox system is in the kinetic region and equals 53 kJ/mol.
The results obtained in this work can be used in the development of technical solutions for treating quarry wastewater from mining enterprises.
References
- Abascal E., Gómez-Coma L., Ortiz I., Ortiz A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Science of the Total Environment. 2022. Vol. 810. N 152233. DOI: 10.1016/j.scitotenv.2021.152233
- Garcia-Zavala C., Ordens C.M., Pagliero L. et al. An approach for prioritising environmental, social and governance (ESG) water-related risks for the mining industry: The case of Chile. The Extractive Industries and Society. 2023. Vol. 14. N 101259. DOI: 10.1016/j.exis.2023.101259
- Jie Hu, Xing Chen, Yeyu Chen et al. Nitrate sources and transformations in surface water of a mining area due to intensive mining activities: Emphasis on effects on distinct subsidence waters. Journal of Environmental Management. 2021. Vol. 298. N 113451. DOI: 10.1016/j.jenvman.2021.113451
- Lai F., Beylot A., Navarro R. et al. The environmental performance of mining operations: Comparison of alternative mining solutions in a life cycle perspective. Journal of Cleaner Production. 2021. Vol. 315. N 128030. DOI: 10.1016/j.jclepro.2021.128030
- Oluwoye I., Dlugogorski B.Z., Gore J. et al. Atmospheric emission of NOx from mining explosives: A critical review. Atmospheric Environment. 2017. Vol. 167, p. 81-96. DOI: 10.1016/j.atmosenv.2017.08.006
- Ferreira H., Mariangela Garcia Praça Leite. A Life Cycle Assessment study of iron ore mining. Journal of Cleaner Production. 2015. Vol. 108. Part A, p. 1081-1091. DOI: 10.1016/j.jclepro.2015.05.140
- Akinnawo S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environmental Challenges. 2023. Vol. 12. N 100733. DOI: 10.1016/j.envc.2023.100733
- Kazapoe R.W., Addai M.O., Amuah E.E.Y., Dankwa P. Characterization of groundwater in southwest Ghana: Implications for sustainable agriculture and safe water supply in a mining-dominated zone. Environmental and Sustainability Indicators. 2024. Vol. 22. N 100341. DOI: 10.1016/j.indic.2024.100341
- Gbedzi D.D., Ofosu E.A., Mortey E.M. et al. Impact of mining on land use land cover change and water quality in the Asutifi North District of Ghana, West Africa. Environmental Challenges. 2022. Vol. 6. N 100441. DOI: 10.1016/j.envc.2022.100441
- Sunitha V., Sudharshan Reddy Y., Suvarna B., Muralidhara Reddy B. Human health risk assessment (HHRA) of fluoride and nitrate using pollution index of groundwater (PIG) in and around hard rock terrain of Cuddapah, A.P. South India. Environmental Chemistry and Ecotoxicology. 2022. Vol. 4, p. 113-123. DOI: 10.1016/j.enceco.2021.12.002
- Lei King, Qiang Wang, Lili Xia et al. Environmental exposure to perchlorate, nitrate and thiocyanate, and thyroid function in Chinese adults: A community-based cross-sectional study. Environment International. 2023. Vol. 171. N 107713. DOI: 10.1016/j.envint.2022.107713
- Kom K.P., Gurugnanam B., Bairavi S. Non-carcinogenic health risk assessment of nitrate and fluoride contamination in the groundwater of Noyyal basin, India. Geodesy and Geodynamics. 2022. Vol. 13. Iss. 6, p. 619-631. DOI: 10.1016/j.geog.2022.04.003
- Stayner L.T., Almberg K., Jones R. et al. Atrazine and nitrate in drinking water and the risk of preterm delivery and low birth weight in four Midwestern states. Environmental Research. 2017. Vol. 152, p. 294-303. DOI: 10.1016/j.envres.2016.10.022
- Hao Su, Yukun Deng, Jiejun Zhao et al. Excellent, steady and economical nitrogen removal in highly variable mining wastewater via three-stage partial-denitrification/partial-nitrification/anammox system: A pilot-scale demonstration. Journal of Water Process Engineering. 2023. Vol. 54. N 103896. DOI: 10.1016/j.jwpe.2023.103896
- Pashkevich M.A., Korotaeva A.E. Evaluation of the efficiency of the phytoextraction process in the quarry wastewater treatment. Mining Informational and Analytical Bulletin. 2022. N 6-1, p. 349-360 (in Russian). DOI: 10.25018/0236_1493_2022_61_0_349
- Ting W.H.T., Tan I.A.W., Salleh S.F., Wahab N.A. Ammoniacal nitrogen removal by Eichhornia crassipes-based phytoremediation: process optimization using response surface methodology. Applied Water Science. 2020. Vol. 10. Iss. 3. N 80. DOI: 10.1007/s13201-020-1163-x
- Rampuria A., Gupta A.B., Brighu U. Nitrogen transformation processes and mass balance in deep constructed wetlands treating sewage, exploring the anammox contribution. Bioresource Technology. 2020. Vol. 314. N 123737. DOI: 10.1016/j.biortech.2020.123737
- Mietto A., Politeo M., Breschigliaro S., Borin M. Temperature influence on nitrogen removal in a hybrid constructed wetland system in Northern Italy. Ecological Engineering. 2015. Vol. 75, p. 291-302. DOI: 10.1016/j.ecoleng.2014.11.027
- Hube S., Zaqout T., Ögmundarson Ó. et al. Constructed wetlands with recycled concrete for wastewater treatment in cold climate: Performance and life cycle assessment. Science of the Total Environment. 2023. Vol. 904. N 166778. DOI: 10.1016/j.scitotenv.2023.166778
- Ying-hua Li, Hai-bo Li, Xin-yang Xu et al. Fate of nitrogen in subsurface infiltration system for treating secondary effluent. Water Science and Engineering. 2017. Vol. 10. Iss. 3, p. 217-224. DOI: 10.1016/j.wse.2017.10.002
- Xuli Zhu, Liang Jiao, Xuan Wu et al. Ecosystem health assessment and comparison of natural and constructed wetlands in the arid zone of northwest China. Ecological Indicators. 2023. Vol. 154. N 110576. DOI: 10.1016/j.ecolind.2023.110576
- Mo Wang, Dong Qing Zhang, Jian Wen Dong, Soon Keat Tan. Constructed wetlands for wastewater treatment in cold climate – A review. Journal of Environmental Sciences. 2017. Vol. 57, p. 293-311. DOI: 10.1016/j.jes.2016.12.019
- de la Varga D., Soto M., Arias C.A. et al. Constructed Wetlands for Industrial Wastewater Treatment and Removal of Nutrients. Technologies for the Treatment and Recovery of Nutrients from Industrial Wastewater. IGI Global, 2017, p. 202-230. DOI: 10.4018/978-1-5225-1037-6.ch008
- Guerrero-Brotons M., Álvarez-Rogel J., Arce M.I., Gómez R. Addressing the C/N imbalance in the treatment of irrigated agricultural water by using a hybrid constructed wetland at field-scale. Journal of Environmental Management. 2023. Vol. 348. N 119329. DOI: 10.1016/j.jenvman.2023.119329
- Hellman M., Hubalek V., Juhanson J. et al. Substrate type determines microbial activity and community composition in bioreactors for nitrate removal by denitrification at low temperature. Science of the Total Environment. 2021. Vol. 755. Part 1. N 143023. DOI: 10.1016/j.scitotenv.2020.143023
- Goyburo-Chávez C., Mendez-Ruiz J.I., Jiménez-Oyola S. et al. Pilot-scale reverse osmosis treatment of gold cyanidation effluent for the removal of cyanide, heavy metal(loid)s, and ionic species. Case Studies in Chemical and Environmental Engineering. 2024. Vol. 9. N 100688. DOI: 10.1016/j.cscee.2024.100688
- Samaei S.M., Gato-Trinidad S., Altaee A. Performance evaluation of reverse osmosis process in the post-treatment of mining wastewaters: Case study of Costerfield mining operations, Victoria, Australia. Journal of Water Process Engineering. 2020. Vol. 34. N 101116. DOI: 10.1016/j.jwpe.2019.101116
- Grossi L.B., Magalhães N.C., Araújo B.M. et al. Water conservation in mining industry by integrating pressure-oriented membrane processes for nitrogen-contaminated wastewater treatment: Bench and pilot-scale studies. Journal of Environmental Chemical Engineering. 2021. Vol. 9. Iss. 1. N 104779. DOI: 10.1016/j.jece.2020.104779
- Bing Wang, Chunyang Gao, Xingchun Li et al. Remediation of groundwater pollution by in situ reactive zone: A review. Process Safety and Environmental Protection. 2022. Vol. 168, p. 858-871. DOI: 10.1016/j.psep.2022.10.046
- Sakr M., El Agamawi H., Klammler H., Mohamed M.M. A review on the use of permeable reactive barriers as an effective technique for groundwater remediation. Groundwater for Sustainable Development. 2023. Vol. 21. N 100914. DOI: 10.1016/j.gsd.2023.100914
- Yangmin Ren, Mingcan Cui, Yongyue Zhou et al. Zero-valent iron based materials selection for permeable reactive barrier using machine learning. Journal of Hazardous Materials. 2023. Vol. 453. N 131349. DOI: 10.1016/j.jhazmat.2023.131349
- Singh R., Chakma S., Birke V. Performance of field-scale permeable reactive barriers: An overview on potentials and possible implications for in-situ groundwater remediation applications. Science of the Total Environment. 2023. Vol. 858. Part 1. N 158838. DOI: 10.1016/j.scitotenv.2022.158838
- Kumarasinghe U., Kawamoto K., Saito T. et al. Evaluation of applicability of filling materials in permeable reactive barrier (PRB) system to remediate groundwater contaminated with Cd and Pb at open solid waste dump sites. Process Safety and Environmental Protection. 2018. Vol. 120, p. 118-127. DOI: 10.1016/j.psep.2018.09.003
- Suzuki T., Moribe M., Oyama Y., Niinae M. Mechanism of nitrate reduction by zero-valent iron: Equilibrium and kinetics studies. Chemical Engineering Journal. 2012. Vol. 183, p. 271-277. DOI: 10.1016/j.cej.2011.12.074
- Yiping Zhang, Douglas G.B., Long Pu et al. Zero-valent iron-facilitated reduction of nitrate: Chemical kinetics and reaction pathways. Science of the Total Environment. 2017. Vol. 598, p. 1140-1150. DOI: 10.1016/j.scitotenv.2017.04.071
- Marcos-Hernández M., Cerrón-Calle G.A., Ge Y. et al. Effect of surface functionalization of Fe3O4 nano-enabled electrodes on the electrochemical reduction of nitrate. Separation and Purification Technology. 2022. Vol. 282. Part A. N 119771. DOI: 10.1016/j.seppur.2021.119771
- Glushankova I.S., Bessonova E.N., Blinov S.M. et al. Removal of nitrogen compounds from mine process water using redox barriers. Mining Informational and Analytical Bulletin. 2021. N 10, p. 58-68 (in Russian). DOI: 10.25018/0236_1493_2021_10_0_58
- Panasyugin A.S., Teran A.I., Grigorev S.V. et al. Joint extraction of nitrate ions and of iron ions by the filter material obtained on base of steel slag. Foundry production and metallurgy. 2018. N 4 (93), p. 32-37 (in Russian). DOI: 10.21122/1683-6065-2018-4-32-37
- Jinghui Zhang, Zhiwei Hao, Zhen Zhang et al. Kinetics of nitrate reductive denitrification by nanoscale zero-valent iron. Process Safety and Environmental Protection. 2010. Vol. 88. Iss. 6, p. 439-445. DOI: 10.1016/j.psep.2010.06.002
- Fang Zhao, Jia Xin, Mengjiao Yuan et al. A critical review of existing mechanisms and strategies to enhance N2 selectivity in groundwater nitrate reduction. Water Research. 2022. Vol. 209. N 117889. DOI: 10.1016/j.watres.2021.117889
- Zhenwei Liu, Shangshang Dong, Di Zou et al. Electrochemically mediated nitrate reduction on nanoconfined zerovalent iron: Properties and mechanism. Water Research. 2020. Vol. 173. N 115596. DOI: 10.1016/j.watres.2020.115596
- Bartzas G., Komnitsas K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. Journal of Hazardous Materials. 2010. Vol. 183. Iss. 1-3, p. 301-308. DOI: 10.1016/j.jhazmat.2010.07.024
