Increasing the efficiency of rare earth metal recovery from technological solutions during processing of apatite raw materials
- 1 — Ph.D. Assistant Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 2 — Ph.D., Dr.Sci. Head of Department Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 3 — laboratory assistant Saint Petersburg Mining University ▪ Orcid ▪ Elibrary
- 4 — laboratory assistant Saint Petersburg Mining University ▪ Orcid
Abstract
The issues of complex processing of mineral resources are relevant due to the depletion of available raw materials. So, it is necessary to involve technological waste, generated during the processing of raw materials, to obtain valuable components. In the process flow of apatite concentrate treatment using the sulfuric acid method, a large amount of phosphogypsum is produced with an average content of light rare earth metals (REMs) reaching 0.032-0.45 %. When phosphogypsum is treated with sulfuric acid solutions, a part of REMs is transferred to the sulfate solution, from which it can be extracted by means of ion exchange method. The study focuses on sorption recovery of light REMs (praseodymium, neodymium and samarium) in the form of anionic sulfate complexes of the composition [ln(SO4)2]– on polystyrene anion exchanger AN-31. The experiments were performed under static conditions at a liquid-to-solid ratio of 1:1, pH value of 2, temperature of 298 K and initial REM concentration in the solutions ranging from 0.83 to 226.31 mmol/kg. Thermodynamic description of sorption isotherms was carried out by the method based on linearization of the mass action equation, modified for the ion exchange reaction. As a result of performed calculations, the authors obtained the constants of ion exchange equilibrium for Pr, Nd and Sm, as well as the values of the change in the Gibbs energy for the ion exchange of REM sulfate complexes on the AN-31 anion exchanger and the values of total capacity of the anion exchanger. Calculated separation factors indicated low selectivity of AN-31 anionite exchanger for light REMs; however, the anion exchanger is suitable for effective recovery of a sum of light REMs. Based on the average value of ion exchange equilibrium constant for light REMs, parameters of a sorption unit with a fluidized bed of anion exchanger were estimated.
Introduction
Nowadays the issues of complex processing of mineral resources are relevant due to the depletion of available raw materials and the necessity to use technological waste, such as phosphogypsum, red mud [10, 15, 16], etc., as well as the possibility of associated recovery of valuable components without disrupting the key production processes.
Russia places strategic importance on rare earth metals (REMs), which are widely used in such high-tech industries as laser technology, medicine, automotive industry and electronics [6].
In the third quarter of 2020, the top positions in the list of countries that supply REMs to the global market are held by China with a share of 33.04 %, Japan with 19.19 % and the United States with 11.7 %, while Russia supplies to the global market only 6.4 %. The share of Russia in REM sales on the global market in the third quarter of 2020 amounts to 1.58 % [13].
Industrial REM sources include monazite, bastnaesite, euxenite and loparite [7]. One of the methods for processing REM-containing concentrates is sulfuric acid leaching, which results in the formation of sulfate solutions [24]. In sulfuric acid solutions, REMs are present in the form of cationic complexes; however, a decrease in H+ concentration of the solution and an increase in the concentration of sulfate ions enable the formation of anionic REM complexes [20].
One of the alternative sources of REM recovery is phosphogypsum, large amounts of which are generated during the processing of apatite concentrate by means of sulfuric acid technology. In the process of sulfuric acid leaching of phosphogypsum, which contains ~0.32-0.45 % of predominantly light REMs, some of them pass into the sulfate solution. Average chemical composition of phosphogypsum can be described as follows: SO3 – 44.07, CaO – 32.04, Fe2O3 – 1.02, fluorides – 0.20, H2O – 18.00, Al2O3 – 0.40, SiO2 – 1.93, P2O5 (total) – 1.49, Ln2O3 – 0.65, TiO2 – 0.20 %.
The main methods for obtaining REMs and other metals from various raw materials are extraction [1, 14, 25], sorption [11, 21, 26] and precipitation [4, 29] processes. Sorption methods are often used to recover elements from multicomponent complex salt solutions. The use of a specific method depends on process conditions and the overall production workflow [3].
Processing of REM-containing raw materials widely uses sulfuric acid solutions, where due to high concentration of sulfate ions, REMs occur in the form of anionic complexes of the second coordination sphere. REM recovery in the form of sulfate complexes using ion exchange resins has been scarcely covered in the literature. However, conversion of REMs into anionic complexes will allow to increase the efficiency of their recovery, which confirms the relevance of the present study.
REM recovery using cation exchange resins
The authors of paper [32] demonstrate the possibility of using a macroporous strong acid cation exchange resin SQS-6 to adsorb lanthanum and neodymium from phosphoric acid media. The authors examine different parameters that affect the sorption of these metals: the ratio of solution volume to the mass of ion exchange resin, concentration of the acid and metal ion. It is identified that the equilibrium state is reached within 10 minutes, the process is spontaneous, endothermic and accompanied by an entropy increase. The results of REM adsorption are consistent with the Langmuir isotherm model over the entire studied range of concentrations. Desorption of La (III) and Nd (III) was carried out using 1.0 M citric acid solution at pH 4. The sorption capacity of SQS-6 resin for lanthanum and neodymium was 33.55 and 17.3 mg/g, respectively.
In paper [22], a strong acid cation exchange resin Lewatit MDS 200H was used for recovery and separation of rare earth elements (REEs) from solutions, the composition of which was similar to acid mine drainage water. Sorption experiments were performed in ion exchange columns at pH 3.5; the initial solution contained 3.13 mmol/l of REEs (La, Pr, Nd, Sm, Eu, Gd, Dy, Er), 1.17 mmol/l of Al, Ca and Mg impurities and 11.6 mol/l of sulfate anion. Elution of the adsorbent was carried out using 0.02 mol/l of NH4-EDTA (ammonium ethylenediaminetetraacetate). The eluate contained 39.0 mmol/l of REEs and 2.79 mmol/l of metal impurities.
The authors of paper [23] studied sorption recovery of lanthanum, iron (III), aluminum and calcium ions from phosphoric acid solutions by macroporous sulfonic cation exchange resin MTS 1600 under dynamic conditions. It was identified that calcium suppressed sorption of lanthanum and other elements: in its presence, dynamic exchange capacity of the sorbent decreased from 53.5 to 11.8 g/l for lanthanum, for iron and aluminum it tended to zero. During the elution of lanthanum and calcium ions by ammonium nitrate solution, maximum concentration of ions amounted to 5.5 and 9.5 g/l, respectively. The ratio of calcium and lanthanum concentrations in the eluate was two times higher compared to the initial solution of phosphoric acid.
Study [18] focused on the sorption of lanthanum and cerium on chelating ion exchange resins M4195, TP207 and XUS43605 with three different functional groups. The authors estimated the effect of pH value and resin mass on its capacity, examined adsorption thermodynamics and used different kinetic models. It was experimentally determined that the nature of the process was spontaneous and endothermic.
The influence of sulfate and chloride media on REE adsorption by strong acid and iminodiacetic acid resins was studied in paper [27]. For the strong acid resin Lewatit MonoPlus SP 112 with sulfonic acid functional groups, adsorption constants for La, Sm and Er were significantly lower in MgSO4 solutions compared to the values obtained in a MgCl2 solution. It was established that the efficiency of Sm recovery from 0.5 M MgSO4 solution was not affected by a change in the solution pH in the interval from 1 to 3. For the iminodiacetic acid resin Purolite S930Plus, a similar significant decrease in the constants of La adsorption was recorded from MgSO4 solution versus MgCl2 solution, which led to a considerable shift towards higher pH value. For both resins, a decrease in adsorption capacity of the REEs in the sulfate solution also led to a decrease in REE selectivity with respect to the key impurity ions. For example, selectivity of a strong acid resin for Sm over Al was more than two times lower in MgSO4 solution as compared to MgCl2, while the selectivity of iminodiacetic acid resin for La over Mg decreased by an order of magnitude in MgSO4 solution compared to MgCl2 one.
REM recovery using various sorbents
In order to separate light rare earth elements (Ce, La, Nd), the authors of paper [33] proposed to use a series of nanocomposite adsorbents with different ratios of magnetite and carbon black. It was identified that the maximum sorption value was achieved with 20 % of and 80 % of C and reached 370 mg/g, given initial metal concentration of 250 ppm and pH 7. Sorption isotherms, obtained for the most effective material, adequately fitted with the Langmuir model. Calculated thermodynamic parameters indicated an endothermic and irreversible chemisorption mechanism.
The authors of study [31] synthesized a GO/P(NIPAM-MA) cryogel, based on the oxide of graphene and N-isopropyl acrylamide-maleic acid, and used it as an adsorbent for REE recovery from wastewater. The equilibrium adsorption capacity for La3+ was 33.1 mg/g. The use of the Langmuir model provided high correlation coefficients for experimental data, which indicated homogenous REM adsorption on the surface of the adsorbent. The use of the material demonstrated preferential adsorption of La3+ compared to Cu2+, Co2+, Ni2+, Nd3+ and Yb3+, which suggested high selectivity of the synthesized adsorbent for lanthanum.
Paper [19] examines the sorption properties of a new polymer obtained by hydrolysis of poly(diethyl-6-(acrylamido)hexylcarbamoylmethylphosphonate). The maximum capacity Qmax was experimentally determined to be 1.5 mmol/g. The Langmuir equation was used to estimate Qmax and the equilibrium constant KL, which equaled 1.72 ± 0.067 mmol/g and 0.302 ± 0.063 l/mol, respectively. It was identified that capacity of the adsorbent was dependent on a number of different parameters: initial concentration of metals, sorbent mass, pH and ionic strength. The study showed that the polymer was selective for gadolinium in Gd/Ni mixtures.
The authors of paper [34] proposed the use of xanthated chitosan (XC) beads as an effective adsorbent for recovering Ce (III) ions. They studied the factors affecting the efficiency of Ce (III) adsorption, namely pH of the solution, stirring rate, phase ratio and contact time. According to the results obtained, the optimal conditions for the process included temperature of 300 K, pH of 4 and adsorbent mass of 0.02 g. Regardless of the initial metal concentrations, the time to reach equilibrium amounted to 10 minutes. Kinetic studies demonstrated that chemisorption was the limiting stage of the process. Using the Langmuir model, the authors calculated the value of maximum sorption, which under optimal conditions of the process reached 555.6 mg/g. The enthalpy and entropy of sorption were –6.18 kJ/mol and 130.36 J/mol∙K, respectively. The adsorption of Ce (III) was driven by the mechanisms of ion exchange and complexation.
The authors of paper [28] developed a chitosan-based adsorbent modified with polyethylenimine for REE recovery from bauxite residue leachates, characterized by low pH, low REE concentration and presence of other trivalent ions. The study confirmed the efficiency of using the obtained material for separation of La (III) from Al (III), the separation factor reached 3.1. The maximum adsorption of La (III) in the absence of impurity component was 2.015 mmol/g. In binary systems, La (III) demonstrated preferential adsorption due to formation of chelate compounds with the material. The adsorbent was reusable and after four adsorption-desorption cycles had a regeneration efficiency of 90 %.
Problem statement
Processing of by-products and wastes of the chemical industry for the purpose of recovering valuable components is a solution to the problems of rational and environmentally efficient use of mineral resources [2, 5]. Production facilities of the PhosAgro company annually generate large amounts of phosphogypsum, which is the main waste product of phosphate fertilizer production, generated during the processing of apatite ore [17]. Currently, the amounts of phosphogypsum subjected to processing are insufficient, which causes significant damage to the environment [30]. It is feasible to accompany industrial waste processing with associated recovery of valuable components, such as REEs, which will allow to obtain the metals without changing the overall production workflow [9].
The use of anion exchange resins makes it possible to obtain REMs from solutions of complex salt composition and separate them from associated impurities, such as iron and calcium, which are present in phosphogypsum and other REM sources [12].
Separation of metals from the solutions is carried out using sorption units during the processing of raw materials. Units with a fluidized bed of sorbent are used for continuous processes of adsorption and ion exchange [8]. Calculation of the ion exchange unit implies determination of basic dimensions of the sorption device, which is based on the use of sorption equilibrium constants, obtained experimentally from sorption isotherms of equilibrium parameters.
This study focuses on sorption recovery of praseodymium, neodymium and samarium from sulfate solutions in the form of anionic complexes in order to determine the value of ion exchange equilibrium constant, based on which technological calculation of the ion exchange unit with a fluidized bed of AN-31 anion exchanger was performed for the recovery of light REMs.
Methodology
Neodymium, praseodymium and samarium were recovered from sulfate solutions in the form of $[\mathrm{Ln{(SO_4)}_2]^-}$ [20] на анионообменной смоле АН-31 (Россия). Перед экспериментом анионит переводили в сульфатную форму.
anionic complexes [20] on anion exchange resin AN-31 (Russia). Before the experiment, anion exchanger was converted to the sulfate form.
AN-31 is a gel anion exchanger with a polystyrene matrix, crosslinked with divinylbenzene (DVB), with active functional groups of secondary and tertiary amines. The average bead size is 0.3-1.25 mm; according to the certificate, its exchange capacity for Cl– is no less than 1.28 equiv/kg.
Anion exchanger AN-31 is a widely used and available ion exchange resin with a low cost, which is an important factor for further implementation of the proposed method of REM recovery into the process chain of the plant. Earlier studies on other anion exchange resins, such as D-403 [20], Cybber EV009, etc., demonstrate low recovery rates and necessitate analysis of new ion exchange resins for industrial use.
Sorption was performed under static conditions at temperature of 298 K for 5-6 h in a GFL thermostated mixing cabinet (Germany) at a constant background content of sulfate ions of 1 mol/kg and REM concentration in the solutions ranging from 0.83 to 226.31 mmol/kg. REM content in the solution was estimated by spectrophotometric method with arsenazo III and X-ray fluorescence analysis using Epsilon 3 spectrometer by PANanalytical (Netherlands
Determination of optimal conditions for REM recovery
Acidity of the sorption medium was estimated based on the rate of REM recovery from the solutions at pH from 1 to 4 and liquid-to-solid ratio of 1:1 (10 cm3 of anion exchanger and 10 ml of the solution). Obtained dependences of recovery rate on pH of the solution are presented in Fig.1, a. The maximum observed values of recovery rate on the AN-31 anion exchanger were 52.8 % for Sm at pH 4 and 59.7 % for Pr at pH 2; hence, pH 2 was chosen as the optimal value for conducting the experiments.
To determine the optimal liquid-to-solid ratio, experiments were performed at pH 2 for the ratios 1:1, 1:5, 1:10, 1:25, 1:50 and 1:100. Fig.1, b shows the dependence of the recovery rate on the phase ratio for samarium and praseodymium.
Recovery rate on the AN-31 anion exchanger at ratios of 1:50 and 1:100 did not exceed 0.01 %. Based on the experimental data, the optimal ratio was chosen to be 1:1
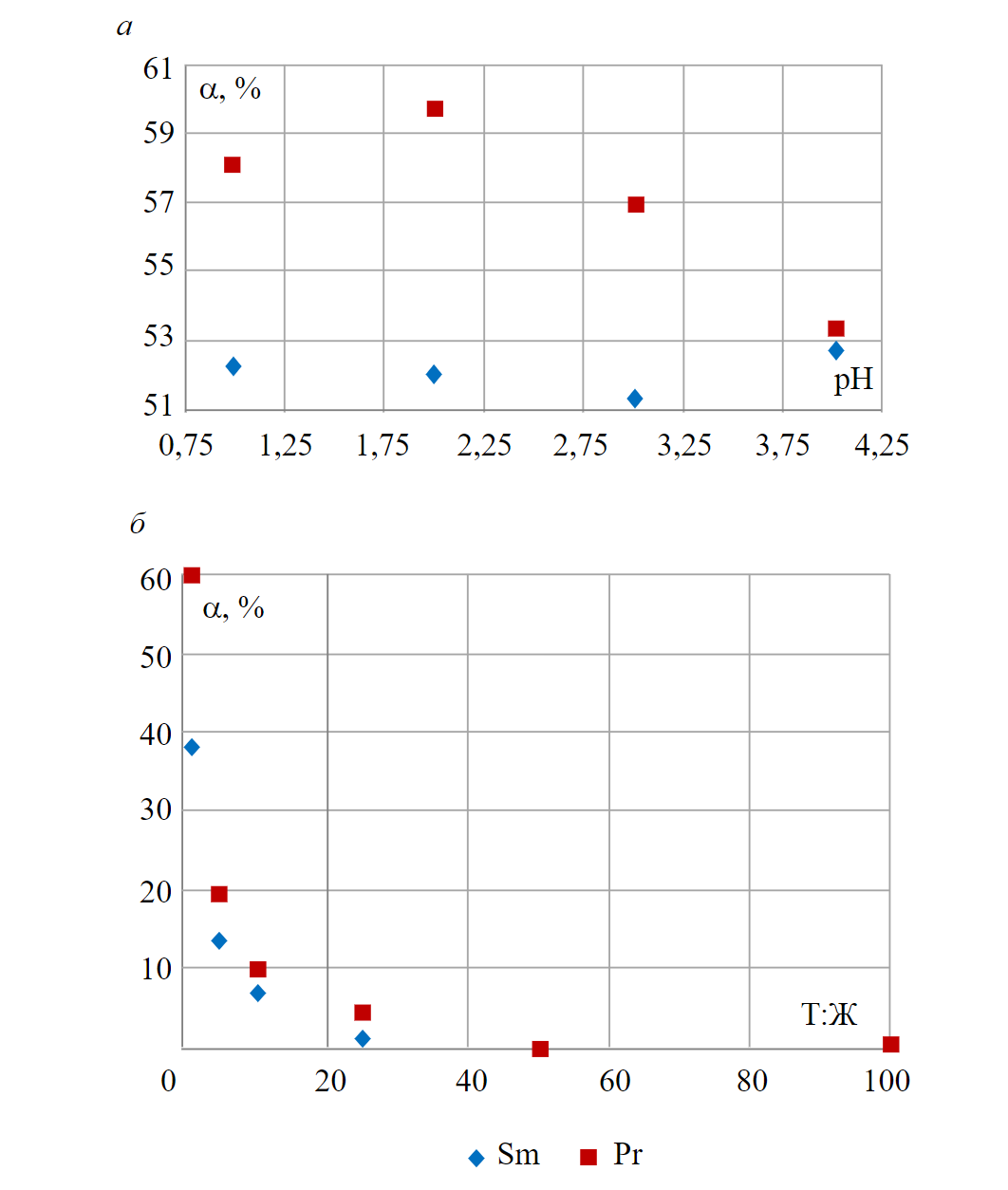
Fig.1. Dependence of the rate of samarium and praseodymium recovery from sulfate solutions on the AN-31 anion exchanger on the pH value of the initial solution (а) and on the solid-to-liquid ratio (b)
Recovery rate on the AN-31 anion exchanger at ratios of 1:50 and 1:100 did not exceed 0.01 %. Based on the experimental data, the optimal ratio was chosen to be 1:1.
Discussion
Calculation of the share of sulfate REM complexes of the second coordination sphere in the solution
Thermodynamic calculation confirms the presence of anionic sulfate complexes of praseodymium, neodymium and samarium in sulfate solutions [20]. Complexes are formed in two stages:
Newly formed complexes are characterized by instability constants KN1 and KN2 for reactions (1) and (2), respectively:
Mathematical transformation allows to obtain a formula for calculating the share of anionic sulfate complexes of REMs in the solution:
where $γ\mathrm{Ln(SO_4)}_2^-$, $γ\mathrm{Ln(SO_4)}^+$,$γ\mathrm{(SO_4)}^{2-}_4$ ,$γ\mathrm{Ln}^{3+}$ – are the activity coefficients of the respective ions; KN1 = 1.63 ∙10–4; KN2 = 7.72 ∙10–3.
Activity coefficients of individual ions were calculated by extrapolating approximate values of the reference data published by L.Meites.
The obtained share of anionic sulfate complexes of praseodymium, neodymium and samarium reached 99.1-99.4 %.
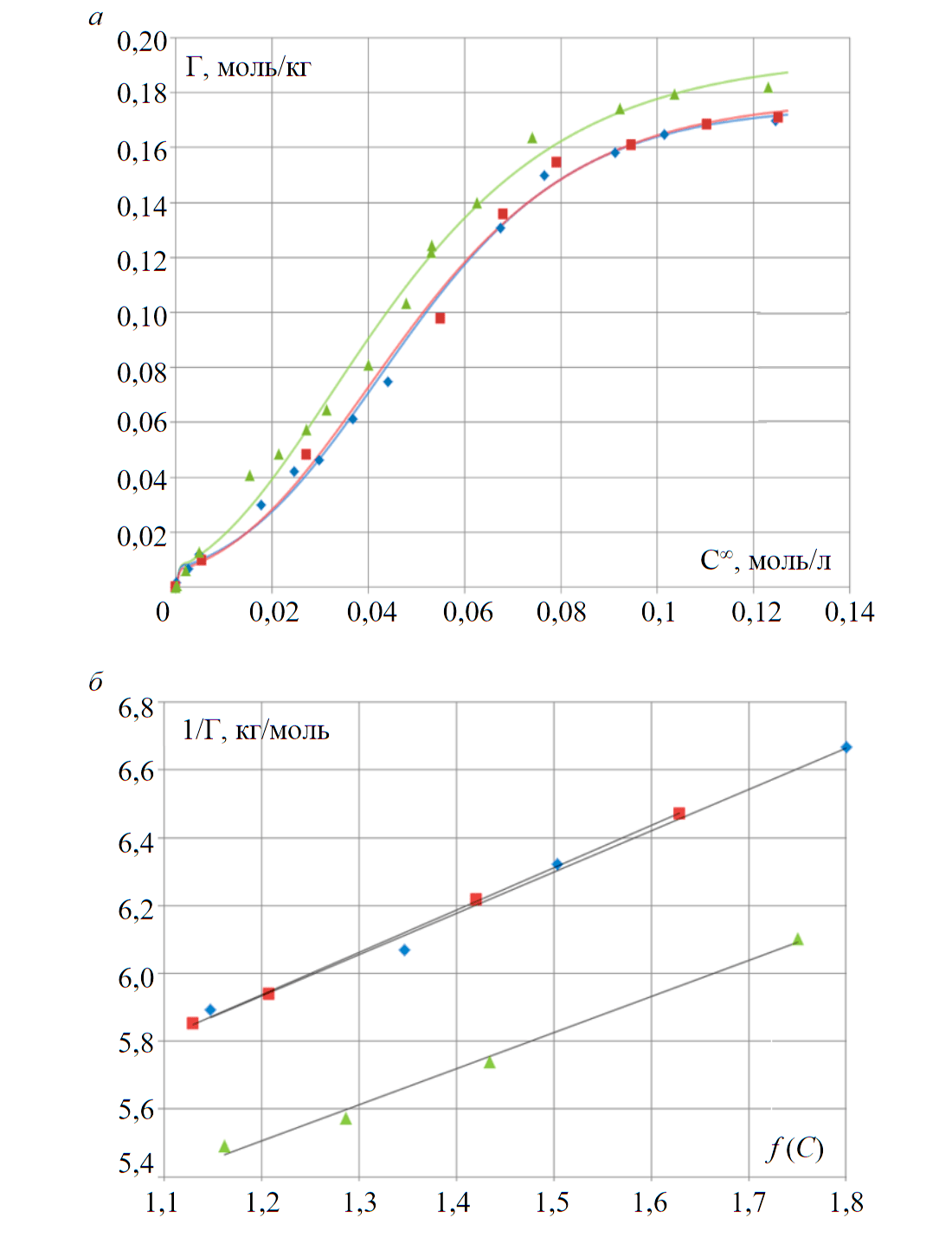
Fig.2. Isotherms of sorption (a) and linear isotherms (b) of praseodymium, neodymium and samarium on the AN-31 anion exchanger
Determination of equilibrium sorption parameters of light REMs
The value of sorption (Fig.2, а) was calculated by the formula:
where С0 and С∞ are the values of initial and equilibrium REM concentration in the solution, mol/l; V is the solution volume, ml; m is the mass of anion exchanger, g; ρ is the solution density (1.13 g/cm3).
The values of ion exchange equilibrium constants and the change in the Gibbs energy on the anion resin were calculated by means of thermodynamic modeling based on linearization of the mass action equation, modified for the ion exchange reaction [20]:
For the process of ion exchange described by formula (3), the mass action equation takes the form:
where $\mathrm{q_{[Ln(SO_4)_3]^{3-}}}$,$\mathrm{q_{SO_4^{2-}}}$ – are the concentrations of respective ions in the solid phase of ion exchange resin, mol/kg; $\mathrm{γ_{±MgSO_4},γ_{±Mg[Ln(SO_4)_2]_2}}$ – are the average ionic activity coefficients of respective electrolytes.
The thermodynamic model assumes ideality of the solid phase and, as a consequence, the value of activity coefficients in the solid phase is taken to be one. Considering weak dependence of average ionic activity coefficients of individual ions on their nature, as well as their main dependence on the charge and ionic strength, the values of activity coefficients for $\mathrm{γ_{±MgSO_4},γ_{±Mg[Ln(SO_4)_2]_2}}$ were calculated using,$\mathrm{γ_{±MgCl_2}}$ to approximate the reference data.
After mathematical transformation of the mass action equation, the following linear form was obtained:
Linear forms of Pr, Nd and Sm isotherms, shown in the coordinates of inverse sorption $\mathrm{\frac{1}{q_{Ln(SO_4)_{2^-}}}}$ and concentration argument , are presented in Fig.2, b.
Approximation equations of linear forms of sorption isotherms, which can be written as y = kx + b, were used to estimate the constants of ion exchange equilibrium K, change in the Gibbs energy $\mathrm{ΔG_{298}^0}$ and total capacity of the anion exchanger (Table 1):
where b is the intercept in the approximation equation of the isotherm linear form; k is the tangent of the slope angle.
Table 1
Thermodynamic characteristics and total capacity of the AN-31 anion exchanger for anionic sulfate complexes of Pr, Nd, Sm
|
REM |
Approximation equation |
Determination coefficient R2 |
Constant of ion exchange equilibrium K |
Change in the Gibbs energy $\mathrm{ΔG_{298}^0}$, J/mol∙K |
Total sorbent capacity q∞, mol/kg |
|
Pr |
y = 1,2172x + 4,4729 |
0,9922 |
1,84±0,09 |
1507,16±73,36 |
0,6707±0,0335 |
|
Nd |
y = 1,2487x + 4,4380 |
0,9995 |
1,66±0,08 |
1259,15±62,96 |
0,6760±0,0338 |
|
Sm |
y = 1,0647x + 4,2278 |
0,9926 |
2,32±0,12 |
2082,96±104,15 |
0,7096±0,0355 |
Obtained values of ion exchange constants and change in the Gibbs energy agree with the data on the sorption of disulfatocerate ions by a weak base anion exchanger D-403: K = 1.77 ± 0.06 and ΔrG298 = –1.42 ± 0.06 kJ/mol [20].
Separation factors are very close to one (D(Sm/Pr) = 1.59; D(Sm/Nd) = 1.21; D(Nd/Pr) = 1.12), which is typical for low REM selectivity of the anion exchanger in the process of element separation, although negative change in the Gibbs energy indicates the effectiveness of using anion exchange resin for recovering a sum of light REMs.
Calculation of a sorption unit with a fluidized bed of AN-31 anion exchanger
According to the data from Table 1, it was estimated that within the margin of error, the average constant of ion exchange equilibrium for a series of light REMs equaled 1.94 ± 0.09. This value was used for technological calculation of the ion exchange unit.
In the calculation of the unit, its throughput in terms of the initial solution was 1.5 m3/h. Initial content of the sum of light REMs in the solution was assumed to be equal to the concentration after the leaching of phosphogypsum with a sulfuric acid solution – 0.0018 mol/l (0.024 %). The key initial parameters for calculating the ion exchange unit included: throughput in terms of the initial solution V – 1.5 m3/h; total capacity of the sorbent for the studied complexes q∞ – 0.6853 mol/kg; average bead size d – 0.71 mm; bulk density of the AN-31 anion exchanger ρbulk – 557 kg/m3; equilibrium constant K – 1.94; initial REM concentration in the solution Cin – 0.0018 mol/l; solution density ρ – 1130.0 kg/m3; porosity of the anion exchanger layer ε – 0.55.
Ion exchange is a process of mass transfer, therefore, calculations need to consider such key parameters as the Reynolds criterion Re = 15.8, which was used to calculate fluid flow rate in the column, the Archimedes number Ar = 1428.7, the mass transfer Nusselt number Nu = 29.4. To determine the limiting stage of diffusion resistance, the mass transfer Biot number Bi = 36.9 was calculated, which demonstrated that the limiting stage of the process was intradiffusion resistance. Table 2 contains calculated values of the sorption column dimensions and dynamic parameters of the process. To select the optimal unit, calculations were performed for a single-section and a multi-section column.
Table 2
Parameters of the anion exchange unit
|
Parameter |
Column |
|
|
Column |
Single-section |
|
|
Unit diameter, m |
0,4 |
0,4 |
|
Number of sections, pcs |
7 |
1 |
|
Operating flow rate of the sorbent, kg/h |
5,1 |
487,3 |
|
Fluidized bed height, m |
0,1 |
0,18 |
|
Fluidized bed height, taking into account separation zone, m |
0,2 |
|
|
Fluidized bed volume, m3 |
0,012 |
0,029 |
|
Fluid flow rate in the column, m/s |
0,0033 |
0,0033 |
Based on the calculation performed, the operating flow rate of the sorbent for a single-section unit reached 487.3 kg/h. To reduce sorbent consumption, calculation of a seven-section unit was carried out, which allowed reducing the operating flow rate to 5.1 kg/h. The flowchart of sorption recovery of a sum of light REMs in a unit with fluidized bed of AN-31 anion exchanger is presented in Fig.3
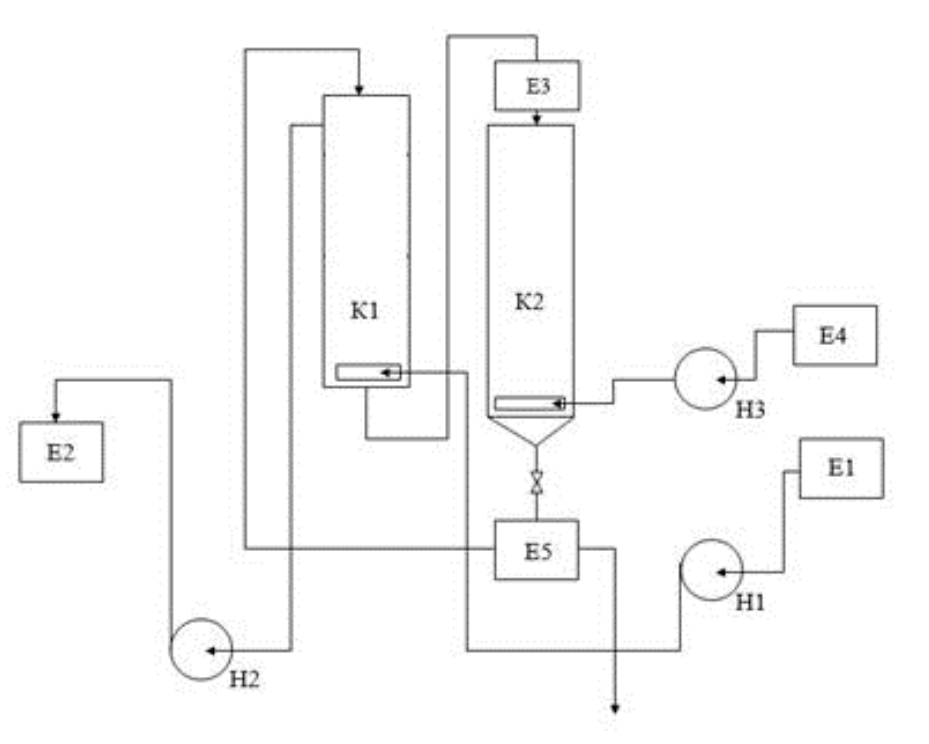
Fig.3. Ion exchange unit E1 –initial solution tank; K1 – anion exchange column; K2 – regeneration column; E2 –clarified water tank; E3 – re-ceiver for spent anion exchanger; E4 –regenerating solution tank; E5 – receiver for regenerated anion exchanger; H1-H3 – pumps
The technological process of recovering light REMs from sulfate solutions, obtained during the processing of phosphogypsum with sulfuric acid, is centered around the sorption column K1 and regeneration column K2. The initial leachate is fed into the E1 tank, where it is prepared for sorption recovery of REMs; after the sorption, the solution is discharged into the E2 tank. By means of an airlift, spent anion exchanger is transferred to the column K2, where it is regenerated with a 2 N solution of sulfuric acid from the tank E4. Regenerated anion exchanger passes into the receiver E5 and returns to the column K1.
AN-31 anion exchanger was regenerated with a 2 N solution of sulfuric acid in two ways: under static and dynamic conditions. In the experiments under static conditions, a portion of anion exchanger (2 cm3) was poured with 50 ml of sulfuric acid solution and left for one day. Under dynamic conditions, sulfuric acid solution was passed through the layer of anion exchanger at a rate of 0.1 rpm. The optimal rate of anion exchanger regeneration reached 89 % under static conditions and 83 % under dynamic ones. REM concentration in the eluate was 0.015 mol/l.
This process flow enables continuous recovery of light REMs from the sulfate solutions of phosphogyspum leaching on the AN-31 anion exchanger.
Conclusions
One of the alternative sources of light REMs is the waste product of phosphate fertilizer production, generated during the processing of apatite raw materials – phosphogypsum, which contains ~0.45 % of REMs. During the processing of phosphogypsum with sulfuric acid solution, a part of REMs is transferred to the solution. As the acidity of the solution lowers to pH 2, REMs form anionic sulfate complexes of [Ln(SO4)2]– composition with a mass fraction of 99.1-99.4 % from the total content of the solution.
The process of sorption recovery of light REMs from sulfate solutions was studied on the example of praseodymium, neodymium and samarium using AN-31 anion exchanger. The experiments were performed under static conditions at temperature of 298 K, liquid-to-solid ratio of 1:1 and contact time of 5-6 h.
Thermodynamic modeling was carried out using the method based on linearization of the mass action equation, modified for ion exchange processes. The values of equilibrium constants, change in the Gibbs energy and total capacity for praseodymium were: K = 1,84 ± 0,09, $\mathrm{ΔG^0_{298}=-1507,16±73,36}$ J/mol, q∞ = 0.67 ± 0.03 mol/kg; for neodymium: K = 1,66 ± 0,08, $\mathrm{ΔG^0_{298}=-1259,15±62,96}$ J/mol, q∞ = 0.68 ± 0.03 mol/kg; for samarium: K = 2,32 ± 0,12, $\mathrm{ΔG^0_{298}=-2082,96±104,15}$ J/mol, q∞ = 0.71 ± 0.04 mol/kg.
Obtained values of separation factors D(Sm/Pr) = 1.59; D(Sm/Nd) = 1.21; D(Nd/Pr) = 1.12 suggest low selectivity of anion exchanger for individual REMs. However, due to high sorption capacity, this anion exchange resin can be recommended for recovering a sum of light REMs. Average value of ion exchange equilibrium constant for a group of light REMs reached 1.94 ± 0.09, total sorbent capacity amounted to 0.6853 ± 0.0343 mol/kg. Based on the data obtained, the calculation for the sorption unit with a fluidized bed of AN-31 anion exchanger was carried out. Operational flow rate of the sorbent for a single-section column was 487.3 kg/h, for a seven-section column it was 5.1 kg/h (given column diameter of 0.4 m).
