Features of the mineral and chemical composition of the Northwest manganese ore occurrence in the Highveld region, South Africa
Abstract
The Northwest manganese ore mineralisation is located at a relative distance from traditionally known manganese mining areas in a new manganese-bearing region (Highveld) in the Northwest Province, Republic of South Africa. The ore occurrence was studied on farms: Buchansvale 61 IQ, Weltevreden 517 JQ, Rhenosterhoek 343 JP and Kafferskraal 306 JP. The data obtained from studying the geology of the area pointed out to interests regarding the development criterias for search of similar ore mineralisations in the northwest region of South Africa. The ore occurs predominantly in the form of powdered manganese wad, manganese nodules and crusts, confined to the karstic structures of the upper section of the dolomites. X-ray powder diffraction (XRD), Scanning electron microscopy with energy dispersive link (SEM-EDS) and X-ray fluorescence were utilized to unveil the mineral and chemical composition of the ore samples. The present study therefore presents the results on both chemical and mineral composition of manganese ores, and their depth and longitudinal distribution. Karstic areas causing an increased local thickness of the ore body were identified. The geochemical and microspcopic study of the ores indicates their supergene nature. The main ore minerals includes cryptomelane, lithiophorite, purolusite, hollandite and romanechite associated with impurity components of Ba, Ce, Co, La, Cr, Zn and V.
Introduction
A new manganese ore occurrence was discovered in the northwestern province of the Republic of South Africa, localized the weathering crust of Neoarchean manganese-bearing dolomites of the Malmani subgroup, Transvaal Supergroup [16, 17, 23]. Ore occurs in the form of manganese nodules, wad and crusts [14]. Karstic processes developed in the area of the ore occurrence determine the relief of the lower boundary of the ore deposit with the Malmani dolomites. Manganese wad forms part of the basal Waterval saprolite preserved in the karstic structures in the upper part of the weathered dolomite section (Fig.1).
Manganese nodules are confined to the alluvial part (Westwits alluvium) of the ore section, and overlie the Waterval saprolite with a sharp erosion contact, characteristic of African I surface of erosion ascribed to regional uplift post-Gondwana.
The ore mineral composition and other chemical features of manganese ore from the Northwestern ore occurrence are still poorly investigated. In particular, the mechanisms of ore formation and bearing sediments, geochemical and mineralogical compositions remains poorly constrained [24, 25]. In this light, the present study is aimed at studying features of ore mineralogy and chemical composition by means of modern analytical methods. Studies of ore minerals indicated the presence of high valence state of manganese (Mn+4). They contain significant amounts of isomorphic admixtures of micro-and rare-earth elements (REE) [1, 18, 27]. REE ore saturation has aroused interest in its mineralogical composition and geochemical characteristics.
Geological structure of the ore occurrence
The Highveld region is located along the northern flank of the Kaapvaal Craton. The area mainly composed of a thick Neoarchean carbonate sequence of the Malmani subgroup and subordinate outcrops of quartzites of the Black Reef series. Depositional basin of the Transvaal Supergroup, to which the Malmani subgroup belongs, indicates the existence of an intracratonic sag basin. A possible reason for its formation is the subsidence of the underlying the Ventersdorp Supergroup deposits under the thermal influence of mantle plumes [21].
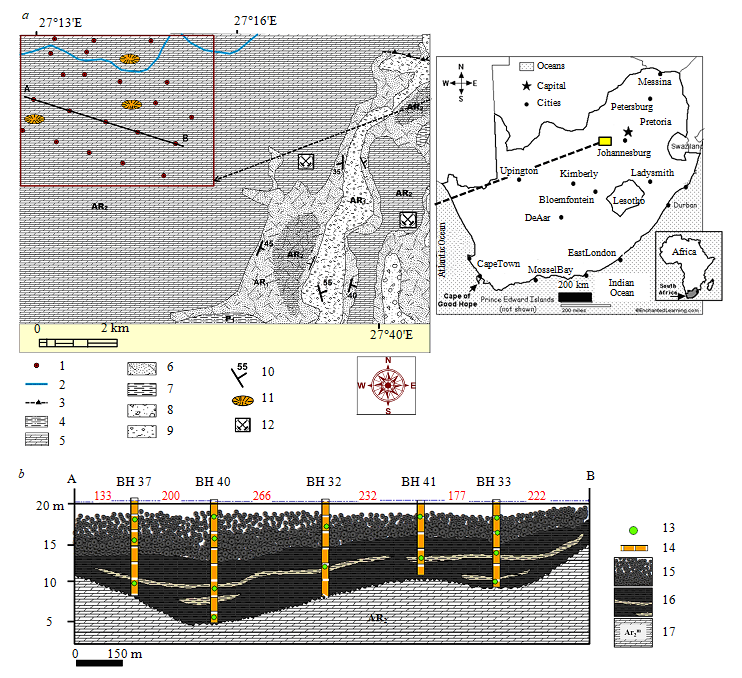
Fig.1. Geological structure (a) and cross-section of the North-Western ore occurrence of Mn, South Africa (b)
1 – exploration pits; 2 – paleodolines; 3 – faults; 4 – siltstone (Р1); 5 – manganese-bearing dolomites of the Malmani series (Ar2m); 6 – quartzite of the retinue of the Black Reef (Ar1); 7 – siltstone with interbedded sandstones (Ar1); 8 – gold-bearing conglomerates with interbedded sandstones(Ar1); 9 – unsorted terrigenous sediments, conglomerates and sandstones (Ar1); 10 – elements of occurrence; 11 – karst funnel(P): depth 6-20 m; 12 – mines – places of field development; 13 – sampling points; 14 – exploration pits; 15 – manganese nodules (N); 16 – powdered manganese wad with a intercalants of clays (K2?); 17 – manganese-bearing dolomite of the Malmani series (Ar2m)
It is known that manganese oxide mineralization in the Highveld region is associated with ancient erosional surfaces – African I and African II, which began to develop in the Late Cretaceous in the post-Gondwana period [4, 32-34].
The tectonic structure of the region is determined by the development of graben-induced depressions and eroded horsts [6]. The average surface relief is 1,550 m above sea level. The relief is formed by an ancient tertiary system of river paleodolines arranged in a fan-shaped pattern. The paleodolines are composed of alluvial deposits framing the proposed watershed [10, 13]. The watershed runs between the rivers draining into the Indian and Atlantic Oceans, and extends from Johannesburg to Lichtenburg [8, 22, 28]. This palaeo-watershed is a relic of the weathered ancient post-Gondwana landmass of the Highveld region and consists of neo-Archean manganese-bearing carbonates, manganese nodules and wad, as well as outcrops of quartzite [5, 14, 15] (Fig.2).
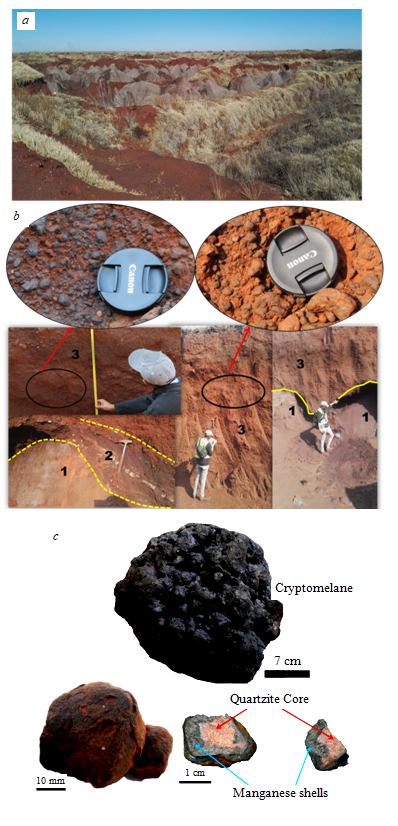
Fig. 2. Field photos: type of the study area (a), geological section (b) and samples of manganese nodules (c)
1 – weathered manganese-bearing dolomite; 2 – manganese wad with a layer of clays; 3 – manganese nodules in alluvial deposits
Tectono-magmatic events in the region are indicated by the presence of Proterozoic magmatic intrusions and, to a lesser extent, by the impact metamorphism of rocks caused by the Vredefort meteorite impact [13]. Uplifts forming graben structures are shown on the Rz-256 seismic profile which runs through the carbonate platform of the Malmani subgroup Ar2m (Fig.3).
Research methodology
Geological exploration was carried out in the Highveld covering most of Mn mineralisation in the north-western province of South Africa. An exploration grid consisting of 70 exploration pits intersecting mineralized bodies was developed at the Buchansvale 61 IQ. At the first stage of the study, 30 samples from five exploration wells were studied (Fig.4). 20 thin sections and 15 polished sections were prepared for studying rocks and minerals under petrographic microscope both in plane polarized and reflected light. During material preparation, the ore samples were first washed with distilled water and dried in a furnace for approximately 24 hours. Parts of the sample were cut off with a diamond saw, glued onto slides, and smoothed using finer abrasive chips to a thickness of 30 microns (thin sections). Polished sections of ore samples were glued together in cups with a diameter of 22 mm and a height of 10 mm. They were additionally polished to the desired smoothness of the polished section surface. This method involved the use of a Michel – Levy interference color diagram.
Cutting and preparation of samples for analytical work was performed using the following devices: Struers (Secotom-10), Struers (Accutom-50).
Identification of mineral phases and phase ratios was performed on an XRD 6000 Shimadzu X-ray diffractometer at the Collective Use Center (CUC) of the Saint Petersburg Mining University. To identify the morphological and structural-chemical properties of the samples, optical and electron microscopy methods of 20 thin sections and 15 polished sections were used (Table 1). Petrographic studies were performed on an Olympus BX-51 microscope. Morphological features of samples were studied using scanning electron microscopes JSM-6460LV and JSM-7001F in the modes of secondary electrons and composite contrast. Chemical analyses were performed using X-ray fluorescence spectrometry on an XRF MagiFast OCP-MS spectrometer. The electron microscopes were equipped with X-Act and X-MAX80 energy dispersive spectrometers, respectively. The analysis of the results was performed at the CUC of the Saint Petersburg Mining University.
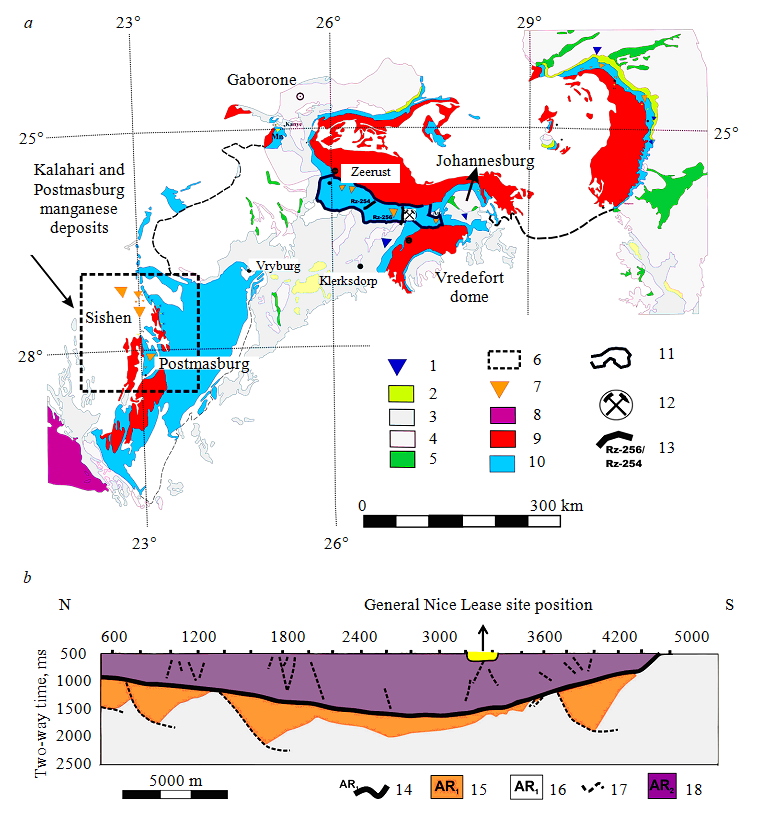
Fig.3. Geological map of the main Archean stratigraphic units of the Kaapvaal Craton according to [7] (a), seismic profile Rz-256 (b)
1 – Au mineralization; 2 – basal layer of volcanogenic-sedimentary rocks (Wolberg group, Ar2); 3 – pre-Transvaal platform (Ar1) (Pongola, Hayes, Witwatersrand, and Ventersdorp supergroups sequence); 4 – archean granites (Ar12); 5 – archean greenstone belts (Ar11); 6 – Kalahari and Postmasburg manganese deposits (Pr11); 7 – mineralization of Mn; 8 – Namaqua granite and metamorphic complexes (K1); 9 – clastic sedimentary units of the upper part of the cut (Pretoria and Postmasburg, T2); 10 – carbonate deposits (Chunispoort and Kembellrand-Griquatown groups, Ar2); 11 – the boundary of the Northwestern manganese ore occurrence presented in this study; 12 – location of the General Nice Mine;
13 – seismic profiles passing through the Malman carbonate platform; 14 – Black Reef series quartzite (Ar13); 15 – Ventersdorp volcanic rocks (Ar12); 16 – granites, foundation gneiss (Ar11); 17 – faults; 18 – manganese-bearing dolomites (Ar2m) [31]
Analyses of 31 impurity elements (Table 2) in 22 ore samples were performed on a Perkin Elmer NexioN 300X inductively coupled plasma (IC-PMS) quadrupole mass spectrometer at the University of Johannesburg. Based on the obtained data, anomalous concentrations of rare-earth elements were determined, which were later normalized to the average compositions of Chondrite and post-Archean Australian shale (PAAS). Based on the obtained analytical results, correlation coefficients and ratios between the contents of manganese, micro- and rare-earth metals were calculated, and anomalous concentrations of elements – genetic markers for determining the source of ore manganese – were established (Table 3).
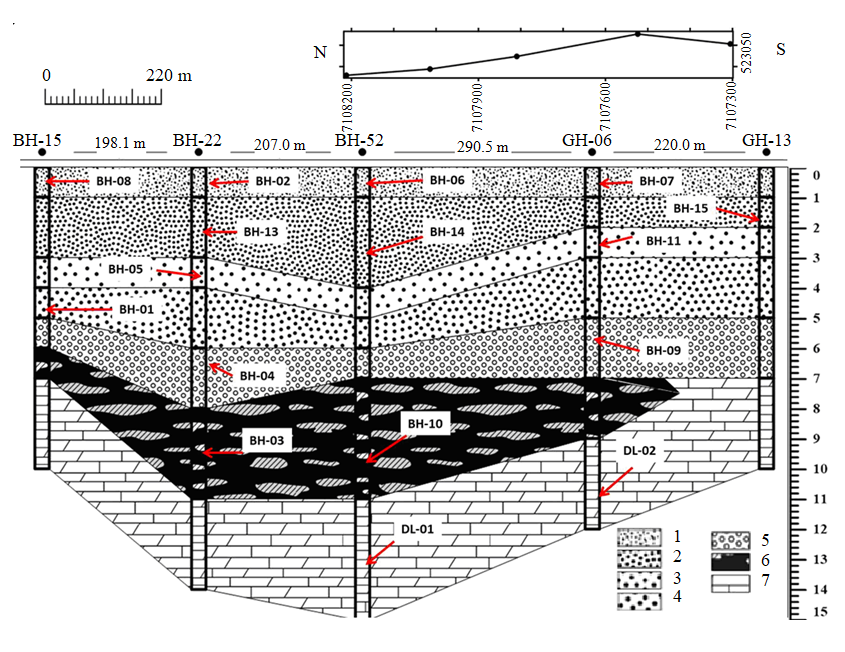
Fig. 4. Geological cut of the North-Western ore occurrence, compiled from wells GNR-BH-15, GNR-BH-22, GNR-BH-52, GN-06 and GN-13
1 – fine-concretion manganese ores of the upper layer; 2 – fine-medium-concretion manganese ores; 3 – medium-concretion manganese ores; 4 – large-concretion manganese ores; 5 – large-concretion manganese ores with a calcrete layer in alluvium; 6 – manganese wad; 7 – manganese-bearing dolomites
The anomalies Ce(CeSN / Ce*SN ) and Eu (EuSN / Eu*SN ) are calculated as coefficients of normalized values by interpolating the contents of neighboring elements:
where SN is the average composition of the post-Archean Australian shale PAAS [29].
Mineral composition of the ore
The ore forming Mn minerals in the area consist predominantly of a group of minerals with a common crystal structure: $[A^{+(2+)}(Mn^{4+}_{6}Mn^{3+}_{2})O_{16}]$, where A are cations: K+, Ba2+, Pb2+. It is a complex of minerals including: cryptomelane $(K^{+}[Mn^{4+}_{6}Mn^{3+}_{2}]_8O_{16})$, hollandite $(Ba^{2+}[Mn^{4+}_{6}Mn^{3+}_{2}]_8O_{16})$, romanechite ((Ba, H2O)2 [Mn4+, Mn3+]5 O10) and lithiophorite ((Al, Li)Mn4+O2 (OH)2). Pyrolusite (a-MnO2) and vernadite $(Mn^{4+}Fe^{3+}CaNa)(OOH)_2\cdot nH_2O)$ are present in subordinate amounts (Fig.5). Accessory minerals are represented by iron oxides, mainly in the form of hematite (Fe2O3) and goethite [FeO(OH)]. The ore contains inclusions of detrital zircon and ilmenite.

Table 3
Pearson coefficient correlation matrix for REE of ore samples, n = 22 (ICP-MS analysis)
|
|
La |
Ce |
Pr |
Nd |
Co |
Ni |
Y |
Ba |
Cr |
Eu |
Yb |
Ce/Ce* |
Eu/Eu* |
|
La |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Ce |
0.478 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
Pr |
0.518 |
0.899 |
1 |
|
|
|
|
|
|
|
|
|
|
|
Nd |
0.523 |
0.895 |
1.000 |
1 |
|
|
|
|
|
|
|
|
|
|
Co |
0.182 |
0.916 |
0.749 |
0.741 |
1 |
|
|
|
|
|
|
|
|
|
Ni |
0.099 |
0.819 |
0.717 |
0.706 |
0.939 |
1 |
|
|
|
|
|
|
|
|
Y |
0.573 |
0.718 |
0.858 |
0.860 |
0.521 |
0.466 |
1 |
|
|
|
|
|
|
|
Ba |
0.303 |
0.942 |
0.900 |
0.897 |
0.877 |
0.803 |
0.690 |
1 |
|
|
|
|
|
|
Cr |
–0.079 |
0.280 |
0.009 |
0.003 |
0.338 |
0.233 |
–0.074 |
0.210 |
1 |
|
|
|
|
|
Eu |
0.485 |
0.903 |
0.997 |
0.998 |
0.763 |
0.728 |
0.844 |
0.919 |
0.010 |
1 |
|
|
|
|
Yb |
0.522 |
0.879 |
0.990 |
0.990 |
0.722 |
0.690 |
0.909 |
0.875 |
0.015 |
0.986 |
1 |
|
|
|
Ce/Ce* |
–0.362 |
0.257 |
–0.083 |
–0.094 |
0.512 |
0.427 |
–0.236 |
0.181 |
0.752 |
–0.072 |
–0.094 |
1 |
|
|
Eu/Eu* |
–0.492 |
0.175 |
–0.059 |
–0.067 |
0.376 |
0.375 |
–0.390 |
0.269 |
0.262 |
–0.017 |
–0.147 |
0.430 |
1 |
Mn oxide phases occur mainly in the form of thin concentric shells around the cores or fragments of other rocks together with heterogeneous cementats (Fig.6, a, b). The formation of manganese oxide mineral phases indicates an early stage of ore formation, characterized by precipitation of manganese oxyhydroxides from ore solutions. The increased degree of oxidation and pH levels of the basinal solutions ascribed to weathering and dissolution of manganese-bearing dolomites led to prevalence of high valence state manganese oxides (Mn4+) forming at the late stage of oregenesis: lithiophorite, cryptomelane, and romanechite [2, 3]. The formation of minerals at this stage of ore genesis occurred mainly through direct precipitation from manganese-rich aqueous solutions. In particular, the development of romanechite over pyrolusite is observed, apparently caused by a change in the composition of mineral-forming solutions as a result of an increase in their pH (Fig.6, c, d, e).
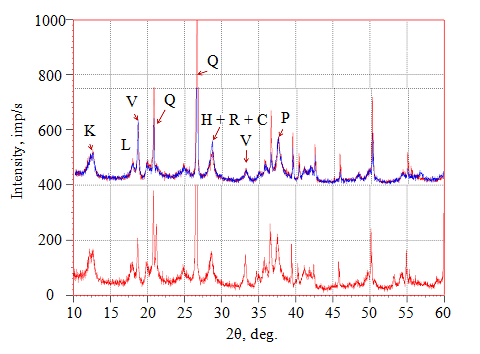
Fig.5. X-ray diffraction spectra of mineral phases in the studied region from two samples:
C – cryptomelane; V – vernadite; R – romanechite; H – hollandite; P – pyrolusite; L – lithiophorite; K – kaolinite; Q – quartz
The process of replacing previously formed mineral phases with manganese oxides is another characteristic feature of the ore. For example, detrital quartz grains in clastic rocks are replaced by cryptomelane. Lithiophorite occurs mainly in the form of thin films with acicular morphology around the pore spaces (Fig.6,f) [9, 11, 30].
To understand the spatial distribution of elements in mineral samples, their element mapping at the microstructural level was performed by scanning electron microscopy (SEM) with energy-dispersive X-ray spectrometry (EDS). Figure 7 shows the profile (A-B) passing through various parts of
the sample. Scanning in the direction of the strike along contacts of ore and nonmetallic minerals, as well as across the intramineral zones, allowed to establish the sequence in which the composition of ore solutions changed. The light areas of the ore (Fig.7, a) correspond to a high concentration of manganese and barium (Fig.7, b, d), while the darker ones are mainly characterized by a high intensity of aluminum, iron, and silica spectra (Fig.7, c, e,f).The positive correlation between the intensities of the manganese and barium spectra reflects the predominance of romanechite. Areas with high concentrations of aluminum, silica, and sodium reflect the composition spectra of clay minerals. The low content of silica, which is evenly distributed over the intramineral zones, is due to the presence of detrital quartz residues and, possibly, chert grains in the manganese oxide cement. The high intensity of the silica spectrum near the end of the profile represents the silicate core of the sample.
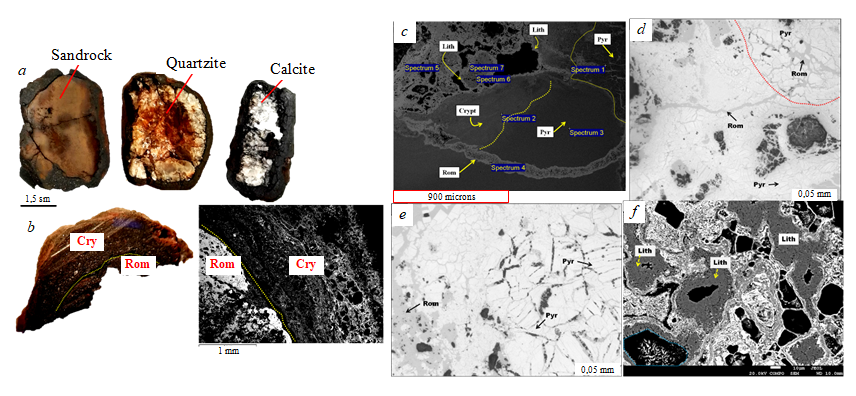
Fig.6. Minerals of manganese oxide ores: a – internal morphological characteristics of manganese nodules, showing different core rocks; b – two adjacent manganese shells, the outer shell consists mainly of cryptomelane (Cry), and the inner shell consists of romanechite (Rom); c – romanechite light gray under reflected light occurs as a vein structure in the groundmass of cryptomelane (Cry) and pyrolusite (Pyr); d and e – diagenetic substitution of pyrolusite and the formation of romanechite due to a change in the redox condition of the medium; e – the occurrence of lithiophorite (Lith) around the pore spaces
Geochemical features of the ore occurrence
The contents of basic and rare-earth elements in ores are shown in Tables 1 and 2. The MnO content ranges from 0.8 to 19 (aver. 12 wt. %). The manganese concentration decreases in the uppermost layer of manganese nodules (TC_08, TC_09; TC_10; TC_11; TC_12; TC_13; TC_14), it has a higher SiO2 content, which is explained by the high content of grains and quartzite fragments in this layer. The upper layer consists mainly of fine-grained manganese nodules.
The content of metal oxides increases in this layer in the following sequence, in weight percent (wt. %): Na2O (0.02-0.1, average 0.05); MgO (< 0.1-0.2, average 0.06); CaO (0.2-3, average 0.09); К2O (0.2-0.9, average 0.60); Fe2O3 (2-20, average10.14); Al2O3(0.3-14.8, average 10.58). Determination of the content of micro-and rare-earth elements in all samples of manganese ores demonstrates a clear enrichment of Ba, V, La, Ce, Ni, Co, Cr, Cu, Zn, and Zr (Table 3; Fig.8). Average content of micro- and rare-earth elements in the ore grow in such a sequence, g/t: Lu (1.24), Tm (1.30), U (3.22), Ho (3.28), Tb (3.68), Cs (4.36), Eu (7.18), Er (8.41), Yb (8.53), TI (10.20), Mo (14.48), Dy (18.87),As (25.07), Gd (26.46), Th (27.18), Sm (30.73), Pr (43.97), Sc (49.06), Zn (52.71), Y (60.32), Nd (157.25), Co (100.93), La (193.67), Pb (212.39), Zr (228.24), Cu (233.65), Cr (229.89), Ni (340.66), V (342.97), Ce (607.64), Ba (10603.55).
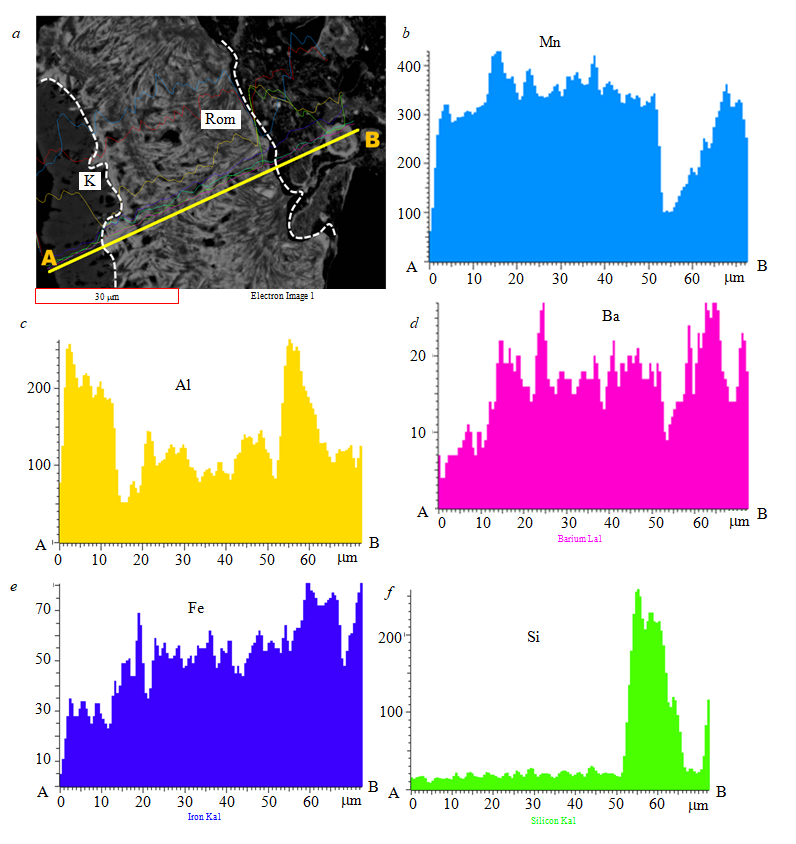
Fig.7. Romanechite (Rom) and kaolinite (K) minerals present in rock samples (a) and elemental mapping of the spatial distribution of the predominant elements: manganese (b), aluminum (c), barium (d), ferrum (e), and silica (f)
Both strong positive and negative correlations are observed between these elements. The Mn content shows a strong positive correlation with the contents of La, Ce, Cu, Ba, Y, Co, Ni, and K, which probably indicates the sorption of these elements from aqueous ore solutions into the tunnel structure of Mn oxides during ore precipitation. A significant similarity is noted in the content of all trace elements, with the exception of Sr (Fig.8, а). Ore minerals may have precipitated from the same ore aqueous solutions.
Another striking feature is the coincidence of the elemental compositions of ore samples and samples of Neoarchean manganese-bearing dolomites of the Malmani subgroup (GNR DD and DOL) (Fig.8, b). In contrast, however, manganese wad represented by sample: WAD indicates a difference in composition of mineral-forming solutions (Fig.8, b).
The Co/Zn ratios in nodules are relatively close in values, varying in the range of 1.01-2.84 (average 2.00), and the Mn wad has a different value – about 0.24. This indicates a higher degree of sorption of micro-and rare-earth elements in nodules than in wad.The Y/HoSN ratios in the ore are increased and range from 7.72-11.78, with an average of 8.81. The La/YbSN ratios vary in the same samples in the range of 1.44-1.98 and are on average 1.65. The coefficients Еu/Eu*SN vary in the range of 1.13-1.19, on average 1.17, and the coefficients Ce/Ce*SN vary 1.13-3.33, on average 1.61.
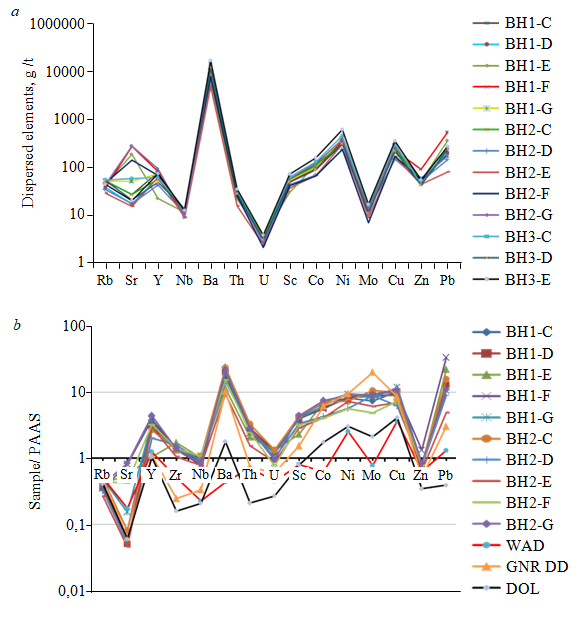
Fig. 8. The content of trace elements (a) and their average contents, normalized by the content of PAAS (b), in ore samples [29]
Conclusion
The northwestern manganese ore occurrence represents near-surface accumulation of secondary manganese oxides in weathering crust underlain by Neoarchean manganese-bearing dolomites of the Malmani series, Transvaal basin. The ore occurrence has the following features:
- The ore mainly consists of manganese wad, nodules and crusts. Manganese wad is preserved in typical karst structures formed as a result of weathering and dissolution of the underlying dolomites. Manganese nodules are confined to the overlying lateritic alluvial part of the ore section.
- The mineral composition of the ore occurrence is mainly characterized by romanechite, cryptomelane, hollandite, pyrolusite, lithiophorite and vernadite. Nonmetallic mineral phases include quartz, calcite, ilmenite and zircon inclusions. Manganese oxide minerals occur mainly in the form of thin concentric accretions around fragments of calcite, quartzite, sand, etc. These mineral associations are similar to those found in other hypergene manganese deposits [12, 20, 35].
- The ore is enriched with a number of rare earth and trace elements: Ce, La, Ba, V, Ni, Cr, Cu and Zr. Most of them positively correlate with the manganese content. The mechanism of sorption of these elements from aqueous ore solutions into the tunnel structure of Mn oxides is assumed [18, 19, 26].
