Gold sorption on modified saponite
- 1 — Ph.D., Dr.Sci. Chief Researcher Institute of Comprehensive Exploitation of Mineral Resources, RAS ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 2 — Ph.D., Dr.Sci. Leading Researcher Institute of Comprehensive Exploitation of Mineral Resources, RAS ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 3 — Ph.D. Senior Researcher Institute of Comprehensive Exploitation of Mineral Resources, RAS ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
Abstract
A potential method for modifying saponite (intercalation) ensuring its high sorption capacity for gold was theoretically and experimentally substantiated. Saponite isolated from recycled water of processing plant tailings dam is modified by mixing a suspension of the mineral and acetone with the surfactant hexadecyltrimethylammonium bromide (CTAB) followed by four washings with ethanol and distilled water, and drying. The intercalation mechanism of saponite-containing product involves the introduction of positively charged cations of organic compounds into the interlayer space through cation exchange or adsorption, which leads to expansion of mineral layers and an abrupt shift in zeta potential toward the positive side. The appearance of bands in the IR spectral ranges of 1460-1490 and 2850-2920 cm–1 related to the deformation and stretching vibrations of the CH2 group, respectively, confirms the successful incorporation of CTAB molecules into the mineral structure. Studies of the maximum sorption capacity of modified saponite revealed that at initial gold concentration 22.6 mg/l, complete extraction is achieved after 7.5 min. The maximum static exchange capacity of modified saponite was achieved after contact with the third portion of fresh gold-bearing solution and amounted to 100.5 mg/g. Gold sorption isotherms correspond to the Langmuir model which presumes that a monomolecular sorption layer forms on the surface of the modified saponite, and all active sites have equal energy and enthalpy of sorption. The kinetic dependences of sorption are best described by a pseudo-second order model, which presumes that the chemical exchange reaction limits the sorption process. It was found that saponite intercalation with hexadecyltrimethylammonium bromide ensures a more efficient sorption of negatively charged gold complex ions ([AuCl4]–). The calculated equilibrium static exchange capacity of modified saponite was 92-119 mg/g, while the experimentally determined value was 102 mg/g.
Introduction
Successful development and modernization of national high-tech industry are possible only with a sufficiently complete supply of strategic mineral raw materials (hydrocarbons, rare and rare earth, noble metals, etc.). The development of environmentally friendly technologies for the efficient extraction of valuable elements from raw materials of different origin is relevant at all stages of processing [1]. For example, gold extraction from productive leaching solutions is achieved by the following main methods: zinc cementation at which the gold complex is reduced to elemental gold (Au) and subsequently precipitated by the introduction of zinc powder; solvent extraction using ionic liquids that are capable of extracting the gold complex from solution in the form of an orga-nometallic compound; the use of activated carbon or ion exchange resin, ensuring the extraction of the gold complex by sorption [2-4]. Activated carbon, used in “carbon in leaching”,“carbon in pulp”, and “carbon in column” processes [5, 6] is most widely applied in gold recovery in industry. However, the sorption of gold complexes on activated carbon is relatively slow due to the slow diffusion of complexes into micropores and their tendency to clog them which leads to reduced sorption capacity (less than 20-30 mg/g) and separation efficiency [7, 8]. This necessitates the search for alternative sorbents.
Smectites are a group of layered silicate minerals belonging to the phyllosilicate (sheet silicate) subclass and possessing a high specific surface area, swelling ability, and cation exchange capacity [9, 10]. Due to their widespread occurrence and unique properties, they are used in different industrial branches and medicine.
A characteristic feature of crystal lattice of smectite group minerals (montmorillonite, beidellite, nontronite, saponite, hectorite) is the ability to introduce a large volume of ions that compensate for (neutralize) the negative structural charge, enabling targeted regulation of their exchange properties [11, 12]. Despite the variable material composition and textural and structural features of natural clays of different geological origin, the Russian and foreign scientists conduct a wide range of studies aimed at modifying the sorption properties of smectites [13-15].
Based on analysis of the scientific and technical literature on modification of clay minerals, it was ascertained that mechanical, thermal, and chemical (organic and inorganic compounds) treatment methods, as well as a combination thereof, are most often used for practical implementation of targeted improvements to their sorption capacity [16-18].
Intercalation (introduction, inclusion) of organic cations compensators into the interlayer space of smectites can occur through extra- and intermicellar exchange and ion adsorption with formation of organoclays. In this case, organic cations compensators, prone to hydration, transform the initially hydrophilic surface of the mineral (e.g., saponite) into a hydrophobic one [19-21]. The possibility to use a wide range of organic compounds also makes it possible to regulate the strength and deformation stability, the optical, sorption, and catalytic properties of clay rocks.
Of numerous options for producing organoclays (in solution, in melt, in situ polymerization), a simpler solution intercalation is most commonly used in practice, allowing an efficient and rapid introduction of polymer molecules into smectite nanolayers [22-24]. Water, alcohol and ketone solutions, chloroform, etc., are used as the liquid phase ensuring an intense intercalation of a larger volume of organic cations compensators [25].
This paper presents the results of studies of the sorption properties of natural saponite, a product of diamond mining wastewater recycling, modified by intercalation of a surface-active organic compound, with respect to gold ions.
Methods
Saponite from the Lomonosovskoe diamond deposit (Arkhangelsk Region, Russian Federation), model and productive gold-bearing solutions were used as research material.
As a result of processing diamond-bearing raw material finely dispersed saponite accumulates in recycled water of the processing plant and is stored at the tailings dam. To extract saponite from the aqueous suspension, a centrifugation method (ULAB, China) was used in the study which allows isolating the source material for producing sorbents with a particle size less than 80 μm (fine fraction) (Fig.1). Saponite content in the solid phase was over 73 % [26, 27]. Cationic surfactant hexadecyltrimethylammonium bromide C19H42BrN (CTAB) which is widely used in various fields of science and industry (biochemistry, molecular biology, nanotechno-logy, medicine and pharmacology, cosmetics and household chemicals) was applied to modify the sorption properties of saponite [28, 29]. Unlike the previous studies [26], the composite was synthesized without preliminary drying as follows: 300 ml of clarified overflow (with solids content 50 g/l) was mixed with 150 ml of acetone (50 % of saponite volume) for one hour. After dispersion, aqueous surfactant solution (4.2 wt.%) was uniformly added to the suspension over the course of 24 h. The suspension was stirred in a water bath (temperature 50 °C) at a constant impeller speed of 300 rpm with an overhead stirrer. After completing the intercalation of surfactant into the saponite structure, the solid fraction was separated in a laboratory centrifuge for 5 min (2500 rpm). To remove Br– ions, the sorbent was sequentially washed in four stages: in the first stage with 200 ml of 50 % ethyl alcohol solution; at subsequent test stages – with 200 ml of distilled water. Drying of the finished sorbent to constant weight was conducted at temperature 50-60 °C in a laboratory muffle furnace (LOIP, Russian Federation) (Fig.2).
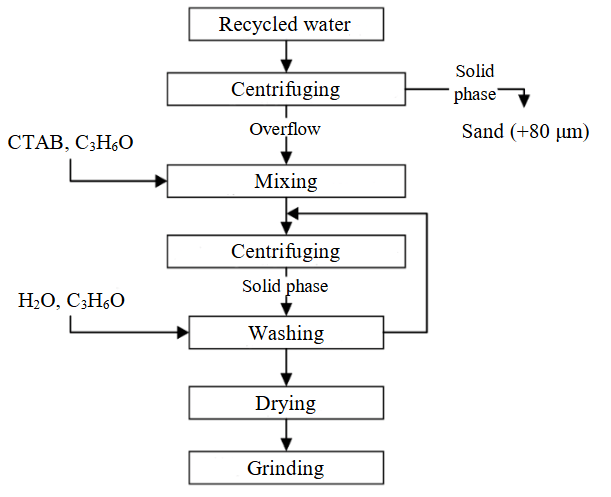
Fig.1. Scheme of producing CTAB-modified saponite
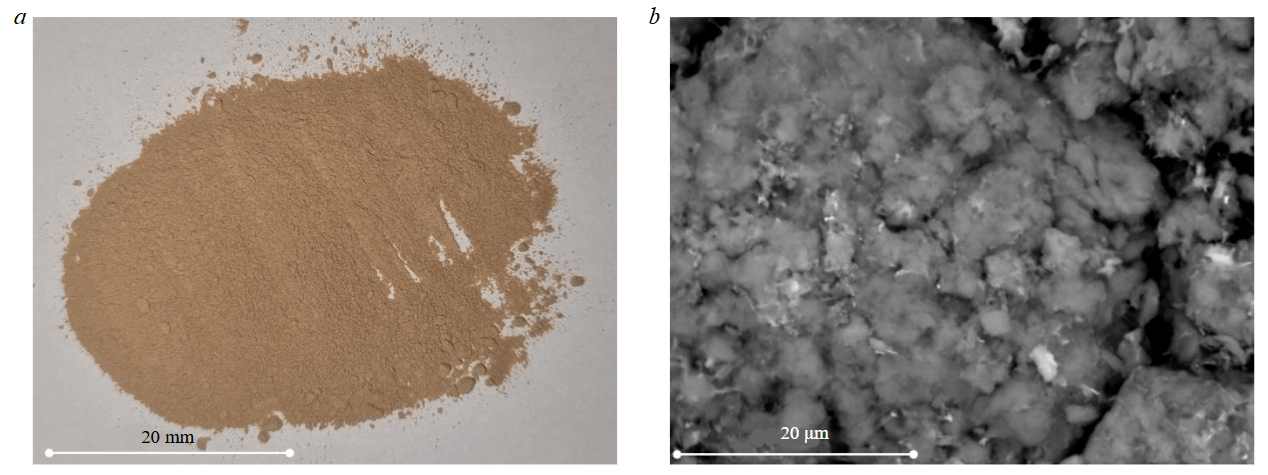
Fig.2. Appearance of CTAB-modified saponite: a – optical microscope; b – electron microscope
Model solutions of HAuCl4 with gold concentration 10-90 mg/l and productive leaching solution of sulphide concentrate from the Vasilkovskoe deposit with gold concentration ~4.5 mg/l were used as gold-bearing solutions [30]. According to thermodynamic modelling data (Hydra, Medusa software), at pH 2.2 of initial model solution, gold ions occur in the form of the AuCl4– complex (Fig.3).
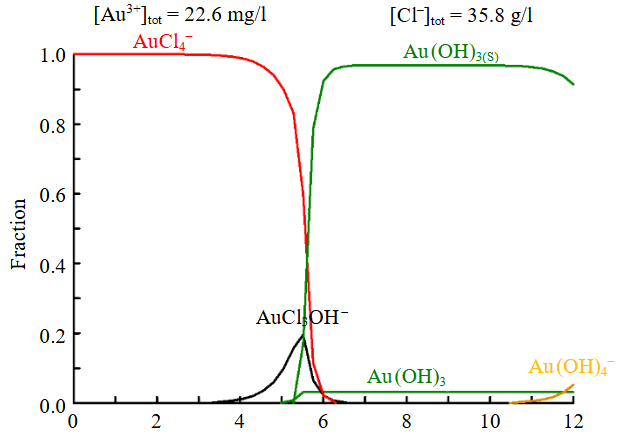
Fig.3. Distribution of gold forms by pH
Gold sorption experiments were performed for 7.5-60 min at modified saponite dosage of 220-500 mg/l using a US-6500 laboratory shaker (ULAB, China) (Fig.4). After completion of sorption, the solution was separated in a UC-4000 laboratory centrifuge (ULAB, China) at acceleration 3500g. Gold concentration in solutions after sorption was determined by a UV-1700 UV spectrophotometer (Shimadzu, Japan) calibrated using standard solutions at a wavelength of 313 nm (R2 = 0.9938) [31, 32].
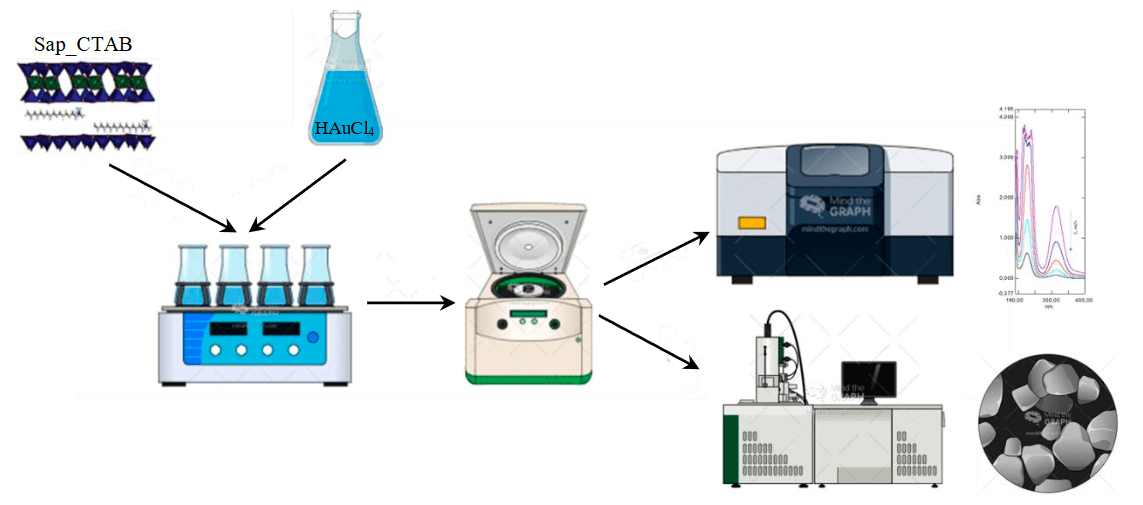
Fig.4. Scheme of experiments on gold sorption
The equilibrium static exchange capacity of modified saponite and gold extraction efficiency were calculated from the following formulas [33]:
where С0, Се are initial and end concentrations of gold in solution, respectively, mg/l; V – volume of solution, ml; m – weight of sorbent, g.
Structural and textural features and material composition of the surface of gold-saturated modified saponite were investigated using a LEO scanning electron microscope (1420VP INCA 350, Zeiss). The interplanar spacing of saponite was determined by X-ray diffractometry (XRD 7000, Shimadzu). The zeta potential of the source and modified saponites was studied in distilled water (pH 6.5) on 0.5 g subsamples after preliminary ultrasonic dispersion using a Nanosizer Zeta Pro analyser (919S, Opptronix). Fourier transform IR spectroscopy was performed on an IR-Affinity spectrometer (Shimadzu). The results were processed by mathematical statistics methods and OriginPro 2018 and Microsoft Excel.
Discussion of results
Substitution of Si4+ by Al3+ and Fe3+ in the tetrahedral sheet and partial replacement of Fe3+ and Al3+ by Fe2+ and Mg2+ in the octahedral sheet explains the negative zeta potential (–41.9 mV) of the source natural saponite. As a result of CTAB intercalation, alkylammonium cations are introduced and absorbed on the surface and in the interplanar space neutralizing the negative charge; the zeta potential of saponite shifts sharply to the positive side to +32.5 mV, which increases the electrostatic attraction and promotes an efficient sorption of anions [34]. According to X-ray phase analysis, after modification, the interplanar distance of saponite increases 1.45-fold from 14.96 to 21.74 Å, which confirms the successful introduction of CTAB molecules into the mineral structure [35, 36].
In IR spectra of modified saponite (Fig.5, a) the appearance of bands in the spectral ranges of 1460-1490 and 2850-2920 cm–1 (Fig.5, b) attributed to deformation and stretching vibrations of the CH2 group, respectively, was recorded [37, 38].
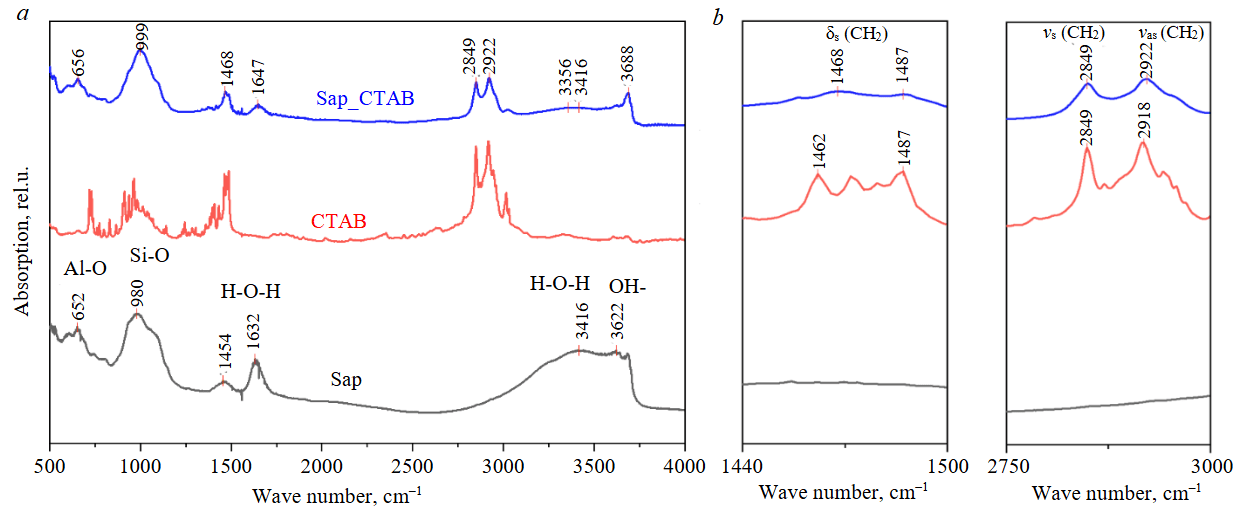
Fig.5. IR spectra of the source saponite (lower spectrum), CTAB (middle spectrum), and saponite after modification (upper spectrum)
Table 1 presents the results of the study of the maximum sorption capacity of modified saponite, a single subsample of the sorbent sequentially contacted with three portions of model solution (each 200 ml in volume) with gold concentration 22.6 mg/l. It was ascertained that upon contact with the first portion of solution, complete gold extraction was attained after 7.5 min. The maximum static exchange capacity of the modified saponite was attained after contact with the third portion of fresh gold-bearing solution and amounted to 100.5 mg/g. As a result of similar studies, it was ascertained that the SEC of the source (unmodified) saponite is only 18.8 mg/g with gold extraction less than 58 %.
Table 1
Results of gold sorption on modified saponite (C[Sap_CTAB] = 500 mg/l; C[Au] = 22.6 mg/l; T = 25 °C)
|
Parameter |
Volume of model solution, ml |
|||||||
|
200 |
+200 (400) |
+200 (600) |
||||||
|
t, min |
0.0 |
7.5 |
7.5 |
15.0 |
30.0 |
7.5 |
15.0 |
30.0 |
|
C, mg/l |
22,6 |
0.0 |
4.5 |
2.5 |
0.0 |
18.2 |
17.3 |
17.3 |
|
ΣE, % |
0.0 |
100.0 |
80.1 |
88.6 |
100.0 |
19.3 |
23.4 |
23.4 |
|
qe, mg/g |
0.0 |
45.2 |
36.2 |
3.9 |
4.7 |
8.7 |
1.8 |
0.0 |
|
Σqe, mg/g |
0.0 |
45.2 |
81.4 |
85.3 |
89.9 |
98.7 |
100.5 |
100.5 |
Figure 6 presents the results of the studies of sorption properties of modified saponite depending on initial gold concentration and sorption duration. It was ascertained that the maximum SEC of CTAB-modified saponite with respect to gold is 91.8-102.4 mg/g.
Adaptation of experimental data to different kinetic models revealed that Au sorption on saponite corresponds to a pseudo-second order model (R2 = 0.99284) assuming that the chemical exchange reaction limits the sorption process [39, 40], and the maximum sorption capacity reaches 119.1±6.7 mg/g (Fig.6, a, Table 2).
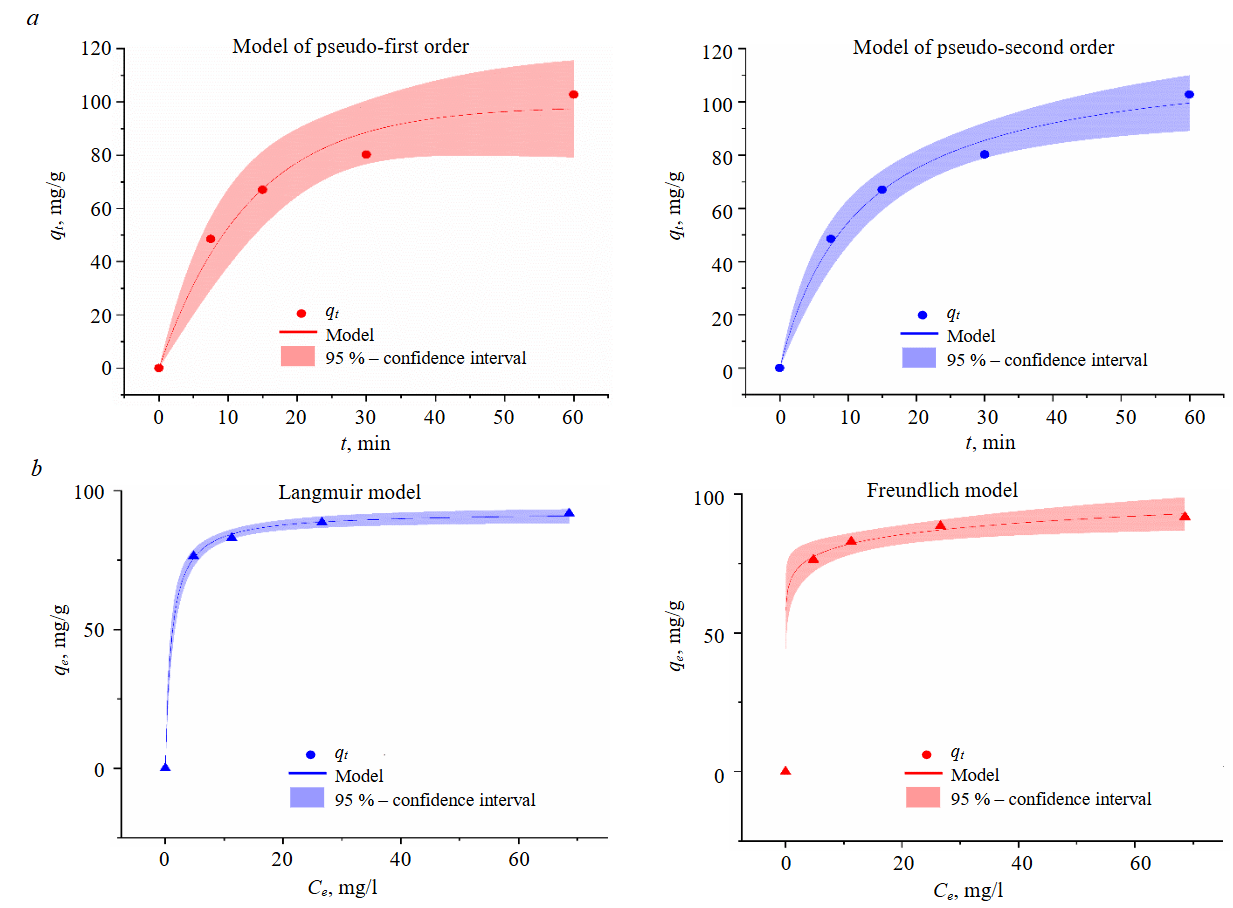
Fig.6. Models of sorption kinetics (C[Au] = 22.6 mg/l; C[Sap_CTAB] = 220 mg/l; T = 25 °C; t = 60 min) (a) and sorption isotherms (C[Sap] = 83-226 mg/l; T = 25 °C; t = 60 min) (b)
Analysis of sorption isotherms revealed that the experimental data correspond to the Langmuir model with the maximum adsorption capacity of 92.3±0.9 mg/g (Fig.6, b, Table 3) based on the fact that a monomolecular sorption layer forms on the surface of saponite, and all active sites have equal energy and enthalpy of sorption [41-43].
Table 2
Kinetic parameters of Au adsorption on modified saponite
|
Model of pseudo-first order |
Model of pseudo-second order |
||||
|
k1, min−1 |
qe, mg/g |
R2 |
k2, g·mg−1∙min−1 |
qe, mg/g |
R2 |
|
7.7∙10–2±1.5∙10–2 |
98.4±6.4 |
0.97876 |
7.1∙10–4±1.6∙10–4 |
119.1±6.7 |
0.99284 |
Note: k1, k2– rate constants.
Table 3
Parameters of Au adsorption isotherms on modified saponite
|
Langmuir model формула
|
Freundlich model |
||||
|
b, l/mg |
qmax, mg/g |
R2 |
nF |
KF, (mg/g)(l/mg)–n |
R2 |
|
0.95±0.1 |
92.3±0.9 |
0.9994 |
0.068±0.009 |
69.7±2.1 |
0.96265 |
Note: b – adsorption coefficient; KF – Freundlich equation constant; nF –dimensionless coefficient.
Data on effective gold sorption by modified saponite are confirmed by analysis of the morphology and chemical composition of the sample surfaces after sorption (Fig.7). Neoformations of different sizes and gold content were detected on the saponite surface after sorption: single large aggregates (Fig.7, d) with Au content 8.5-10.5 % and individual microinclusions (Fig.7, b) with Au content 3.5-8.5 %. Elemental analysis of surface areas not occupied by newly formed phases also revealed the presence of a small amount of gold (from 0.5 to 3.5 %) (Fig.7, a).
Thus, using modern physical and physicochemical methods of analysis, this study confirmed a possibility of efficient modification of the sorption properties of saponite by intercalating the cationic surfactant with hexadecyltrimethylammonium bromide. As a result of the studies of kinetics and sorption isotherms, morphology and elemental composition of modified saponite, a fundamental possibility of obtaining efficient sorbents of gold-bearing complexes on its basis was established, ensuring both a high level of gold extraction and high sorption properties – over 100 mg/g.
Table 4 presents the results of preliminary studies of the sorption properties of CTAB-modified saponite using real hypochlorite leaching solutions of sulphide concentrate from the “Altyntau Kokshetau” processing plant (formerly the Vasilkovskii Mining and Processing Plant) with gold concentration 4.5 mg/l (six portions of real solution, each 200 ml in volume). As with model solutions, the maximum gold ion sorption was recorded in the first 10-30 min, with the maximum static exchange capacity reaching 75 mg/g.
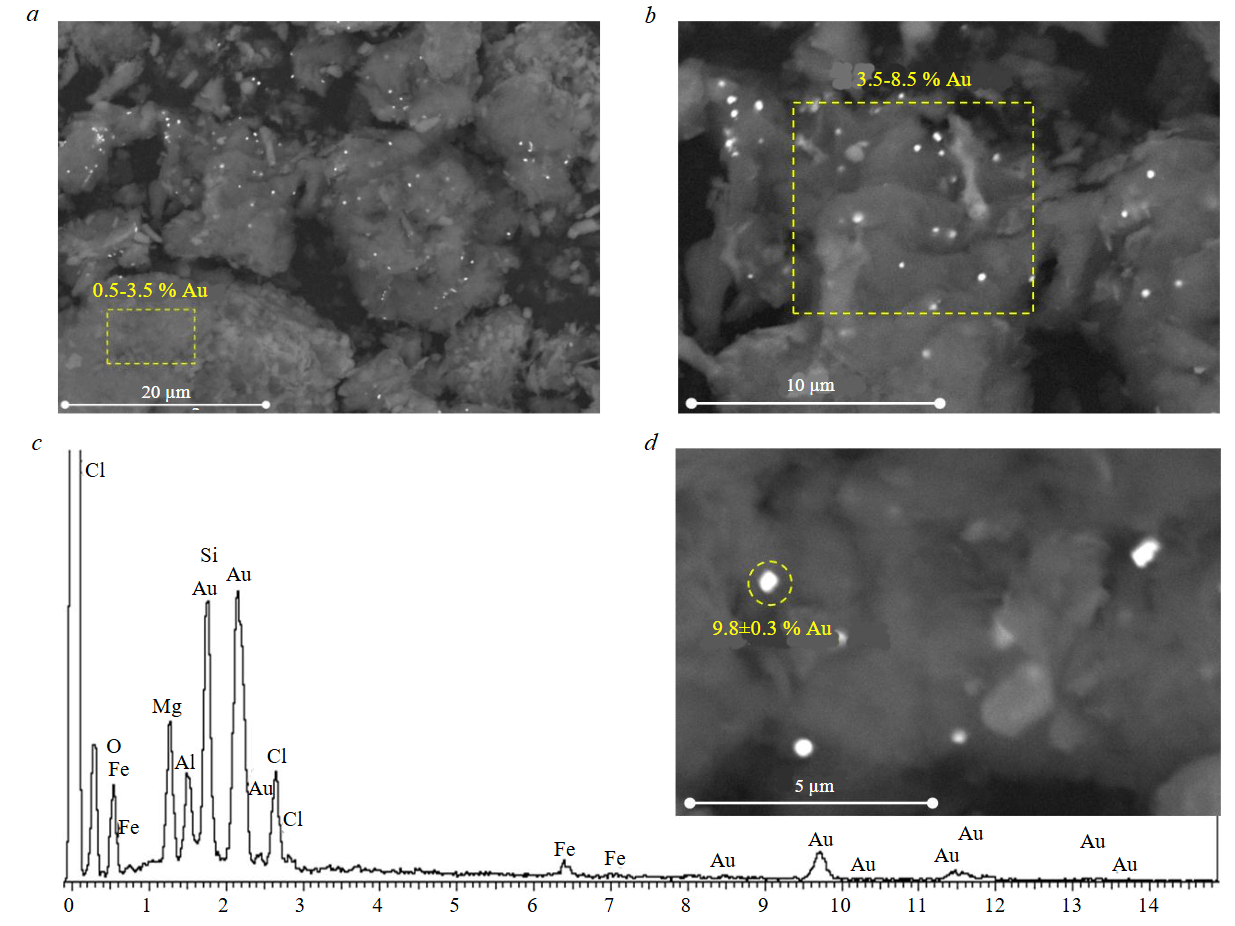
Fig.7. Photomicrographs (a, b, d) of CTAB-modified saponite surface after sorption and an energy-dispersive spectrum (c) of a single gold neoformation (highlighted by a circle)
Table 4
Results of gold sorption on modified saponite (C[Sap_CTAB] = 250 mg/l, C[Au] = 4.5 mg/l, T = 25 °C)
|
Parameter |
Volume of real solution, ml |
||||||
|
200 |
+200 (400) |
+200 (600) |
+200 (800) |
+200 (1000) |
+200 (1200) |
||
|
С0 |
Се |
Се |
Се |
Се |
Се |
Се |
|
|
C, mg/l |
4.5 |
0.0 |
0.0 |
0.1 |
0.3 |
3.425 |
4.5 |
|
ΣE, % |
0.0 |
100.0 |
100.0 |
97.8 |
93.3 |
23.9 |
0.0 |
|
qe, mg/g |
0.0 |
18.0 |
18.0 |
17.6 |
16.8 |
4.3 |
0.0 |
|
Σqe, mg/g |
0.0 |
18.0 |
36.0 |
53.6 |
70.4 |
74.7 |
74.7 |
Conclusion
Sorption properties of CTAB organomodified saponite for gold extraction from productive leaching solutions of gold-bearing concentrate were studied. It was ascertained that CTAB intercalation to 15 wt.% leads to expansion of saponite interplanar space from 14.9 to 21.7 Å, an increase in the zeta potential to positive values from –41.9 to +32.5 mV, and the appearance of peaks characteristic of methylene (CH2) groups in the IR spectra, which ensures a more efficient sorption of negatively charged complex gold ions AuCl4–. It was experimentally determined that gold sorption isotherms on modified saponite with correlation coefficient 0.9994 correspond to the Langmuir model. Moreover, the kinetic dependences of sorption are best described by the pseudo-second order model, assuming that the chemical exchange reaction limits the sorption process. The calculated equilibrium static exchange capacity of modified saponite with respect to gold ([AuCl4]–) was 92-119 mg/g, while the experimentally determined value was 102 mg/g. Preliminary investigations of productive solution of hypochlorite leaching of sulphide concentrate confirmed a high static exchange capacity (75 mg/g) of CTAB-modified saponite which exceeds the declared capacity of the widely used GoldSorb brand activated carbon – to 20-30 mg/g.
Further work will be aimed at studying the regeneration processes of saturated sorbents, the influence of the size (granulation) of saponite and ionic composition of productive solutions on the efficiency of gold extraction.
References
- Chanturia V.A., Nikolaev A.I., Aleksandrova T.N. Innovative Environmentally Safe Processes for the Extraction of Rare and Rare-Earth Elements from Complex Ores of Perplexed Material Composition. Geology of Ore Deposits. 2023. Vol. 65. N 5, p. 425-437. DOI: 10.1134/S1075701523050045
- Nandiyanto Asep B.D., Nugraha Willy C., Yustia Intan et al. Isotherm and kinetic adsorption of rice husk particles as a model adsorbent for solving issues in the sustainable gold mining environment from mercury leaching. Journal of Mining Institute. 2024. Vol. 265, p. 104-120.
- Voropanova L.А., Kokoeva N.B. A Technique for selective extraction of ions of gold and silver from hydrochloric solutions with tributylphosphate. Journal of Mining Institute. 2016. Vol. 222, p. 823-827. DOI: 10.18454/PMI.2016.6.823
- Aleksandrova Т.N., Afanasova A.V., Aburova V.A. “Invisible” noble metals in carbonaceous rocks and beneficiation products: feasibility of detection and coarsening. Mining Science and Technology. 2024. Vol. 9. N 3, p. 231-242. DOI: 10.17073/2500-0632-2024-03-229
- Heshami M., Taheri B. An experimental study on the adsorption behavior of gold glycinate complex on graphene oxide. Hydrometallurgy. 2024. Vol. 224. N 106229. DOI: 10.1016/j.hydromet.2023.106229
- Romero H., Suarez C., Salazar N. et al. Evaluation of gold adsorption on activated carbon from real cyanide and thiourea leachate solutions. Heliyon. 2024. Vol. 10. Iss. 11. N e31606. DOI: 10.1016/j.heliyon.2024.e31606
- Epiforov A.V., Kozlov A.A., Nemchinova N.V., Seleznev A.N. The carbon adsorption of gold from thiocyanate containing sulfuric acid solutions of gold-copper float concentrates atmospheric leaching. Tsvetnye metally. 2020. N 1, p. 38-44 (in Russian). DOI: 10.17580/tsm.2020.01.06
- Jinsong Xia, Marthi R., Twinney J., Ghahreman A. A review on adsorption mechanism of gold cyanide complex onto activation carbon. Journal of Industrial and Engineering Chemistry. 2022. Vol. 111, p. 35-42. DOI: 10.1016/j.jiec.2022.04.014
- Wypych F., Alves de Freitas R. Chapter 1 – Clay minerals: Classification, structure, and properties. Developments in Clay Science. 2022. Vol. 10, p. 3-35. DOI: 10.1016/B978-0-323-91858-9.00004-5
- Shaojian Xie, Lei Huang, Changqing Su et al. Application of clay minerals as adsorbents for removing heavy metals from the environment. Green and Smart Mining Engineering. 2024. Vol. 1. Iss. 3, p. 249-261. DOI: 10.1016/j.gsme.2024.07.002
- Pengsheng Wang, Xinkai Shen, Shusheng Qiu et al. Clay-Based Materials for Heavy Metals Adsorption: Mechanisms, Advancements, and Future Prospects in Environmental Remediation. Crystals. 2024. Vol. 14. Iss. 12. N 1046. DOI: 10.3390/cryst14121046
- Orucoglu E., Grangeon S., Gloter A. et al. Competitive Adsorption Processes at Clay Mineral Surfaces: A Coupled Experimental and Modeling Approach. ACS Earth and Space Chemistry. 2022. Vol. 6. Iss. 1, p. 144-159. DOI: 10.1021/acsearthspacechem.1c00323
- Chun Hui Zhou, Qian Zhou, Qi Qi Wu et al. Modification, hybridization and applications of saponite: An overview. Applied Clay Science. 2019. Vol. 168, p. 136-154. DOI: 10.1016/j.clay.2018.11.002
- Krupskaya V.V., Zakusin S.V., Tyupina E.A. et al. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals. 2017. Vol. 7. Iss. 4. N 49. DOI: 10.3390/min7040049
- Xiaotong Yang, Yi Zhou, Jingjing Hu et al. Clay minerals and clay-based materials for heavy metals pollution control. Science of The Total Environment. 2024. Vol. 954. N 176193. DOI: 10.1016/j.scitotenv.2024.176193
- Najafi H., Farajfaed S., Zolgharnian S. et al. A comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Safety and Environmental Protection. 2021. Vol. 147, p. 8-36. DOI: 10.1016/j.psep.2020.09.028
- Kanglong Cheng, Qin You, Linxi Zou et al. High-temperature calcination modified red clay as an efficient adsorbent for phosphate removal from water. Environmental Research. 2025. Vol. 268. N 120704. DOI: 10.1016/j.envres.2024.120704
- Kabdrakhmanova S., Aryp K., Shaimardan E. et al. Acid modification of clays from the Kalzhat, Orta Tentek deposits and study their physical-chemical properties. Materials Today: Proceedings. 2023, p. 6. DOI: 10.1016/j.matpr.2023.04.427
- Costanza-Robinson M.S., Payne E.M., Dellinger E. et al. Influence of water saturation on interlayer properties of HDTMA-, HDTMP-, and HDPy-modified montmorillonite organoclays. Applied Clay Science. 2024. Vol. 247. N 107188. DOI: 10.1016/j.clay.2023.107188
- Huawen Han, Rafiq M.K., Tuoyu Zhou et al. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. Journal of Hazardous Materials. 2019. Vol. 369, p. 780-796. DOI: 10.1016/j.jhazmat.2019.02.003
- Kotal M., Bhowmick A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Progress in Polymer Science. 2015. Vol. 51, p. 127-187. DOI: 10.1016/j.progpolymsci.2015.10.001
- Shanshan Mao, Manglai Gao. Functional organoclays for removal of heavy metal ions from water: A review. Journal of Molecular Liquids. 2021. Vol. 334. N 116143. DOI: 10.1016/j.molliq.2021.116143
- Perelomov L., Mandzhieva S., Minkina T. et al. The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities. Minerals. 2021. Vol. 11. Iss. 7. N 707. DOI: 10.3390/min11070707
- Khankhasaeva S.T., Badmaeva S.V. Preparation of Nanoporous Sorbent Based on Bentonite Clay and Aluminum Complexes for Use in Water Purification Processes. Protection of Metals and Physical Chemistry of Surfaces. 2024. Vol. 60. N 4, p. 610-617. DOI: 10.1134/S2070205124702150
- Goronja J.M., Janošević-Ležaić A.M., Dimitrijević B.M. et al. Determination of critical micelle concentration of cetyltrimethylammonium bromide: Different procedures for analysis of experimental data. Hemijska industrija. 2016. Vol. 70. Iss. 4, p. 485-492. DOI: 10.2298/HEMIND150622055G
- Chanturia V.A., Minenko V.G. Secondary Products Obtained from Saponite-Bearing Process Water: Theoretical and Experimental Justification. Journal of Mining Science. 2023. Vol. 59. N 6, p. 965-976. DOI: 10.1134/S1062739123060108
- Dvoichenkova G.P., Minenko V.G., Masanov A.Yu., Timofeev A.S. Justification of cryogenic technology for clarification of saponite-containing recycling water of tailings dump processing plant N 1 of JSC Severalmaz. Sustainable Development of Mountain Territories. 2024. Vol. 16. N 2 (60), p. 692-709 (in Russian). DOI: 10.21177/1998-4502-2024-16-2-692-709
- Wei Hua Yu, Ting Ting Zhu, Dong Shen Tong et al. Preparation of Organo-Montmorillonites and the Relationship Between Microstructure and Swellability. Clays and Clay Minerals. 2017. Vol. 65. Iss. 6, p. 417-430. DOI: 10.1346/CCMN.2017.064068
- Zhiping Shi, Pengxiang Li, Liyan Liu. Interactions between CTAB and montmorillonite by atomic force microscopy and molecular dynamics simulation. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2023. Vol. 657. Part B. N 130656. DOI: 10.1016/j.colsurfa.2022.130656
- Chanturia V.A., Samusev A.L., Minenko V.G. Stimulation of Chemical and Electrochemical Leaching of Gold from Rebellious Minerals. Journal of Mining Science. 2020. Vol. 56. N 5, p. 818-827. DOI: 10.1134/S1062739120057166
- Matveeva T.N., Getman V.V., Karkeshkina A.Yu. Flotation and Adsorption Capacities of Dithiopyrilmethane in Gold Recovery from Rebellious Arsenical Gold Ore. Journal of Mining Science. 2020. Vol. 56. N 4, p. 648-653. DOI: 10.1134/S1062739120046934
- Wojnicki M., Luty-Błocho M., Socha R.P. et al. The kinetic studies of gold(III) chloride complex adsorption mechanism from an aqueous and semi-aqueous system. Journal of Molecular Liquids. 2019. Vol. 278, p. 43-52. DOI: 10.1016/j.molliq.2019.01.028
- Chanturia V.A., Minenko V.G., Samusev A.L. et al. Adsorption of Rare Earth Elements at Modified Saponite. Journal of Mining Science. 2024. Vol. 60. N 3, p. 485-493. DOI: 10.1134/S1062739124030153
- Jorge N.L., Garrafa M.V., Romero J.M. et al. Adsorption of Ciprofloxacin on Clay Minerals in Argentinian Santa Rosa-Corrientes Soils. Molecules. 2024. Vol. 29. Iss. 8. N 1760. DOI: 10.3390/molecules29081760
- Qian Li, Min Yi, Lin Shao et al. CTAB modified metakaolin-based geopolymer microspheres for the selective adsorption and recovery of TcO4−/ReO4−. Separation and Purification Technology. 2024. Vol. 350. N 127853. DOI: 10.1016/j.seppur.2024.127853
- Oliveira G.A., San Gil R.A.S., Gonzalez W.A. et al. Synthesis and structural characterization of HPW-doped niobium pillared Brazilian clay. Microporous and Mesoporous Materials. 2024. Vol. 368. N 113030. DOI: 10.1016/j.micromeso.2024.113030
- Moslemizadeh A., Aghdam S.K.-Y., Shahbazi Kh. et al. Assessment of swelling inhibitive effect of CTAB adsorption on montmorillonite in aqueous phase. Applied Clay Science. 2016. Vol. 127-128, p. 111-122. DOI: 10.1016/j.clay.2016.04.014
- Yunyan Zhu, Yuming Cui, Yiming Peng et al. Preparation of CTAB intercalated bentonite for ultrafast adsorption of anionic dyes and mechanism study. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2023. Vol. 658. N 130705. DOI: 10.1016/j.colsurfa.2022.130705
- Jianlong Wang, Xuan Guo. Adsorption kinetic models: Physical meanings, applications, and solving methods. Journal of Hazardous Materials. 2020. Vol. 390. N 122156. DOI: 10.1016/j.jhazmat.2020.122156
- El Abbadi S., El Moustansiri H., Douma M. et al. Enhancing the performance of alumina-pillared clay for phenol removal from water solutions and polyphenol removal from olive mill wastewater: Characterization, kinetics, adsorption performance, and mechanism. Journal of Water Process Engineering. 2024. Vol. 63. N 105432. DOI: 10.1016/j.jwpe.2024.105432
- Jianlong Wang, Xuan Guo. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere. 2020. Vol. 258. N 127279. DOI: 10.1016/j.chemosphere.2020.127279
- Maleki S., Abedi E., Hashemi S.M.B. Insights into kinetic, isotherm, and thermodynamic of ultrasound mode- and amplitude-dependent carotenoid and chlorophyll degradation or/and adsorption. Ultrasonics Sonochemistry. 2024. Vol. 111. N 107130. DOI: 10.1016/j.ultsonch.2024.107130
- Allaoui I., El Mourabit M., Arfoy B. et al. Adsorption equilibrium, kinetic, and thermodynamic studies on the removal of paracetamol from wastewater using natural and HDTMA-modified clay. Desalination and Water Treatment. 2024. Vol. 318. N 100345. DOI: 10.1016/j.dwt.2024.100345
