On the possibility of utilization of carbonate-containing mining waste by producing photocatalytic composite materials
- 1 — Ph.D., Dr.Sci. Head of the Department Belgorod State Technological University named after V.G.Shukhov ▪ Orcid
- 2 — Ph.D. Senior Researcher Belgorod State Technological University named after V.G.Shukhov ▪ Orcid
- 3 — Ph.D. Associate Professor Belgorod State Technological University named after V.G.Shukhov ▪ Orcid
- 4 — Junior Researcher Belgorod State Technological University named after V.G.Shukhov ▪ Orcid
Abstract
Subsurface use waste accounts for the overwhelming majority of waste generated and accumulated in Russia. The increase in the volume of processing of minerals by the mining and processing industries leads to an aggravation of environmental problems – the negative impact of overburden dumps, tailings of enrichment and processing of mineral raw materials on the environment is increasing. Using the example of three types of rocks, the possibility of using carbonate subsurface use waste as raw materials in the formation of photocatalytic composite materials (PCM) in the production of building materials and products, and simultaneously solving environmental problems of territories through large-scale utilization of man-made waste, is considered. A complex of physical (porosity, specific surface area, dispersion, surface morphology) and chemical (chemical composition, acid-base centers, zeta potential, hydrogen index) studies of the properties of carbonate materials of various genetic types have been carried out to determine the possibility of their use as a substrate in the production of PCM. The photocatalytic material obtained by depositing sol-gel synthesized titanium compounds onto a mineral carrier is intended for incorporation into cement building composites and for giving them self-cleaning properties during operation. The mineral powders of limestone from the Tyushevskoye (T) and Porechenskoye (P) deposits and marble from the Polotskoye deposit were ranked according to certain requirements – dispersion, porosity, and adsorption activity. The establishment of numerical indicators for each type of raw material made it possible to determine the degree of suitability of mineral powders of carbonate rocks for the production of composite materials introduced into the composition of building materials. A ranking of mineral powders was carried out to increase the potential efficiency of use in the composition of PCM in the following sequence: limestone T → limestone P → marble. PCM based on carbonate carriers exhibit high rates of organic pollutant degradation (more than 90 %) and are applicable as photocatalytic agents.
Funding
The study was carried out as part of the implementation of the State assignment of the Ministry of Education and Science of the Russian Federation N FZWN-2023-0006 using the equipment of the High Technology Center of BSTU named after V.G.Shukhov.
Introduction
The availability of mineral resources is an important competitive advantage of the Russian economy, which determines the country’s place and role in the international arena. Along with high rates of mining and processing of minerals, the mining and processing industry is one of the main sources of large-tonnage waste, similar in composition and properties to natural raw materials. Subsurface use wastes are represented by overburden and host rocks, sludge, tailings of mineral processing and other wastes from geological survey, exploration, extraction and primary processing of mineral raw materials, containing or not containing minerals and components. According to Rosstat data for 2023*, 8666.3 million t of waste were generated by mining enterprises, of which about 40 % (3564.8 million t) are recycled. The main ways to reduce environmental damage from the release and accumulation of mining waste are to reduce their volume, neutralization and utilization [1].
The need to dispose of subsurface use products by involving waste in industrial use is due to the high environmental burden on the environment [2]. The expansion of the range of technologies for the use of secondary mineral resources in the creation of high-tech products with high added value can significantly contribute to solving the problems of the most complete use of waste and resource conservation.
Rocks of carbonate composition are among the most widespread and demanded types of mineral raw materials used in the production of a wide range of products. During the extraction and processing of carbonate rocks (limestone, chalk, shell limestone, dolomite, marble, calcareous tuff, marls), the proportion of waste generated can be 30-40 % of the total mass of the processed material. Among relatively monomineral rocks, they occupy the third place in the line of raw materials for building materials, behind clays and quartz sands. Among the modifiers of mineral origin, limestone (22 %), blast furnace granular slag (58 %), and pozzolan additive (15 %) are the most widely represented in the market of additive cements. Mineral powders, which act as fillers in building products and structures, compact the structure of concrete, allowing to reduce cement consumption and reduce the cost of the product [3, 4].
To assess the possibility of using carbonate waste as a potential mineral raw material in the production of photocatalytic composite materials (PCM) intended for the production of self-cleaning building materials, studies were conducted where monomineral rocks of various genetic types, such as limestone and marble, were considered as model systems.
The high interest in self-cleaning materials is confirmed by the growth of research into the creation of photocatalytic components and their use in the construction industry [5, 6]. Synthesis [7], modification (doping, sensitization to visible light) [8-10], and the use of an affordable and effective photocatalyst, titanium dioxide, in building materials [11-13], are being studied.
A factor limiting the use of titanium dioxide in concrete products is its inertia with respect to the components of the hardening system, which leads to the weathering of loose photocatalyst particles from the surface of the finished product and a decrease in the efficiency of self-cleaning during operation. The problem of fixing a photocatalytically active component in a building material is proposed to be solved by using a composite material of the “core – shell” type, in which the photocatalyst is previously deposited on a carrier. In this case, the carrier must actively interact with the basic substance of the matrix, have a chemical affinity with it, participate in the processes of structure formation, strengthening the cement-concrete matrix of the material.
The analysis of foreign and domestic studies has shown positive results of the synthesis and introduction of PCM into the composition of building products, in which silica [14-16] and aluminosilicate raw materials [17-19] are used as photocatalyst carriers. The possibility of using carbonate materials has been less studied [20]. As for silica and aluminosilicate compositions, when using carbonate carriers, it is possible to achieve activation of the transformation of photogenerated electron-hole pairs on the surface of the photocatalyst as a result of the interaction between TiO2 and CaCO3, as well as an increase in the degree of adsorption of pollutants for their subsequent decomposition [4].
Carbonate rocks are widespread in the Earth’s crust and occur as waste from the mining and processing industry [21-23], the effectiveness of their use in finely dispersed form in new generation concrete compositions has been proven [24-26]. A large number of carbonate rocks deposits makes it possible to expand the mineral resource base for the production of photocatalytic composite materials and, consequently, the range of building materials and products with self-cleaning effects. The use of mining and processing industry waste in composite materials will expand the range of technologies for the utilization of carbonate raw materials in the production of high-tech products. In this regard, it is important to study the possibility of photocatalyst deposition on carbonate materials.
The requirements for silica carriers of a photocatalytic agent for use in cement systems have been established: high SiO2 content (more than 70 %), carrier dispersion in the range of 0.1-500 mm with polymodal particle distribution, the presence of mesopores 2-50 mm in size, high activity rates of Lewis and Bronsted adsorption centers on the carrier surface [27].
The paper presents the results of studying the complex of physical-chemical properties of carbonate materials of various genetic types as model systems for ranking the effectiveness of using a photocatalytic agent as a carrier in a composite material of the “core – shell” type for involving carbonate raw materials of both natural and man-made origin in the PCM production technology.
Methods
Types of carbonate materials. Powdered, finely dispersed carbonate materials of various genetic types used in the composition of concrete as mineral additives of sedimentary chemogenic origin were used in the work – limestone T (OOO Stromis, Lipetsk Region, Tyushevskoye deposit), limestone P (OOO Tsentr-Izvestnyak, Tula Region, Porechenskoye deposit); metamorphic origin – marble dust MD as a waste from grinding and fractionation of natural marble (OOO Rif-micromramor, Chelyabinsk Region, Magnitogorsk, Polotskoye deposit). All types of carbonates have a white color, which allows them to be used in mixtures with white cement.
Carbonate materials were studied in the condition in which they were obtained from suppliers and used in the production of building materials. Thus, the use of these types of mineral powders makes it possible to minimize the cost of additional preparation (by grinding) of the carrier during the PCM preparation.
Sol-gel deposition of a photocatalytic agent on a carrier. Photocatalytic composite materials were synthesized using sol-gel technology to pre-verify the ranking of carbonate rocks in terms of the effectiveness of using a photocatalytic agent as a carrier. Sol compositions and technological parameters of sol-gel synthesis were developed earlier [28]. The technology consists in mixing a titanium precursor in a spirit solvent in the presence of a carrier, as a result of which new formations of titanium compounds are fixed on its surface, and subsequent temperature treatment, which promotes the crystallization of titanium hydrates and oxyhydrates into anatase. As a result of structural transitions, a “photocatalyst – carrier” composite material is formed, capable of decomposing complex organic pollutants into simple ones when irradiated with ultraviolet light.
The chemical and mineral compositions of carbonate materials were studied using an ARL 9900 WorkStation X-ray fluorescence spectrometer and an ARL X’TRA X-ray diffractometer (Cu-anode). When measuring the chemical composition, the error in determining the amount of oxides that make up the materials under study is within ±0.25 wt.% for CaO and ±0.1 wt.% for the other oxides. X-ray phase analysis was used to determine the qualitative content of minerals in the composition of carbonate raw materials, and therefore quantitative measurement errors were not taken into account.
The particle size was determined by laser diffraction using an Analysette 22 NanoTec plus laser analyzer.
The specific surface area was assessed by the method of breathability on the PSKh-11M(SP) device, the specific active surface and porosity at the nanoscale were assessed by the method of low-temperature nitrogen adsorption/desorption using the BELSORP-MINI X device.
The pH value of an aqueous suspension of carbonate materials was determined by the potentiometric method using an Orville ML-51 digital pH meter. When determining the pH of aqueous suspensions of powdered materials, three parallel measurements were carried out, the error between the obtained values did not exceed 2-3 %.
The electrokinetic potential (ζ-potential) of the particle surface charge (at a concentration of 1 g/l of raw material) was studied electrophoretically using a Zetatrac laser analyzer.
The concentration and distribution of acid-base centers on the surface of carbonate materials were detected using a spectrophotometric method for evaluating the adsorption of indicators from an aqueous medium with a pKa in the range from –4.4 to 12.8 on a UNICO 2802S spectrophotometer.
The microstructural features of carbonate materials were studied using a Tescan MIRA 3 LMU scanning electron microscope.
The photocatalytic activity of powder materials was assessed by the degree of degradation of the organic pollutant [29] rhodamine B (an aqueous solution with a concentration of 4∙10–4 mol/l) according to the UNI 11259 method at an ultraviolet irradiation intensity of 2.7 W/m2 using the GIMP 2.10.8 program.
Photocatalytic composite materials were synthesized using the developed sol-gel technology, where the studied carbonates were introduced into the reaction mixture as a photocatalyst carrier.
The photocatalytic ability of materials is confirmed, according to the UNI 11259:2016 standard, if the condition of discoloration of Rx rhodamine B is more than 20 % after 4 h of ultraviolet irradiation and more than 50 % after 26 h of irradiation from the initial value of the color coordinate a of the Lab color space, characterizing the position of the color on the axis between red and green.
Two milliliters of an aqueous solution of rhodamine B was applied to the prepared samples of powdered materials. After keeping in the dark for 30 min, the samples were exposed to ultraviolet radiation. Before and after 4 and 26 h of exposure, the colorimetric method was used to determine the coordinate a and calculate the degree of discoloration of the dye:
Results discussion
The presence of impurities in the composition of the carbonate carrier can affect both the nature of the deposition of the photocatalytic agent on it and the interaction of PCM based on it with the cement binder during hydration. In this regard, a comparative assessment of the compositions of carbonate raw materials was carried out.
Table 1
Chemical and mineral composition of the studied materials
|
Material |
Content of oxides, wt.% |
||||||||||||
|
СаO |
SiO2 |
Al2O3 |
MgO |
Fe2O3 |
SO3 |
K2O |
Na2O |
SrO |
TiO2 |
CO2 |
l.o.i.* |
||
|
Limestone T |
52.2 |
2.82 |
1.10 |
0.92 |
0.44 |
0.17 |
0.12 |
0.08 |
0.04 |
0.03 |
42 |
0.08 |
|
|
Limestone P |
53.4 |
1.51 |
0.68 |
0.90 |
0.23 |
0.08 |
0.06 |
0.07 |
0.05 |
0.01 |
43 |
0.01 |
|
|
Marble dust MD |
55 |
0.12 |
0.06 |
0.31 |
0.02 |
– |
– |
– |
0.02 |
0.002 |
43 |
1.47 |
|
* l.o.i. – losses on ignition.
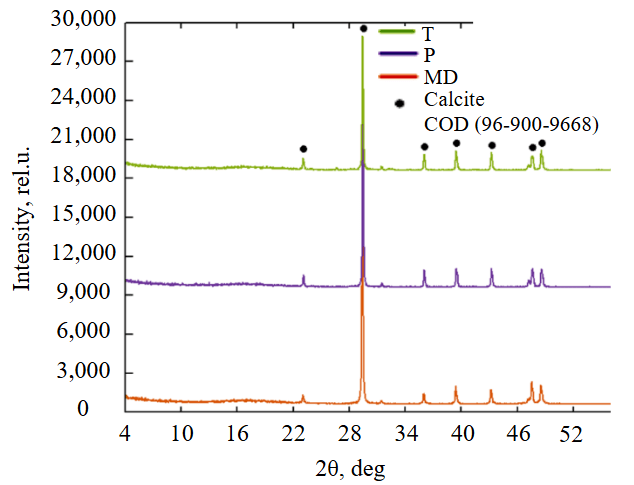
Fig.1. Mineral composition of the studied materials
The chemical and mineral composition of the studied materials (Table 1, Fig.1) is typical for carbonate sedimentary and metamorphic rocks, the content of CaO is 52-55 wt.%, CO2 is 42-43 wt.%, i.e. more than 90 % is represented by calcite. Most of the impurity oxides SiO2, Al2O3, MgO, Fe2O3 are observed in the composition of limestone T, which can diversify the composition of acid-base centers on its surface. Marble dust is characterized by the purest chemical composition. Iron impurities can stain rocks, affecting the appearance of the synthesized photocatalytic composite material.
Carbonate additives are introduced into cement composites as fillers to optimize the fractional composition and create the densest packaging [30] and as a component involved in hydration processes that affect changes in the phase composition of cement stone and increase frost resistance [31, 32]. In both cases, the level of dispersion of additives is in the range of 400-500 m2/kg [33] and is comparable to the rational values applied to carriers when preparing PCM, therefore, the characteristics of the carbonates under consideration were evaluated.
The nature of the particle size distribution has been determined by laser diffraction (Fig.2). Limestones are characterized by a polymodal particle distribution. The particle sizes of limestone T are in the range of 0.15-195 μm (peak values at 13-17, 85-105 μm), limestone P – 0.1-135 μm (peak values at 2.5-4, 13-17, 65-75 μm) (Fig.2, a, b). Marble dust has three peak values of 1.5-2.5, 9-14, and 60-65 μm (Fig.2, c). Among the studied carbonate materials, marble dust is characterized by the highest dispersion, and limestone T is characterized by the lowest (Fig.2, d).
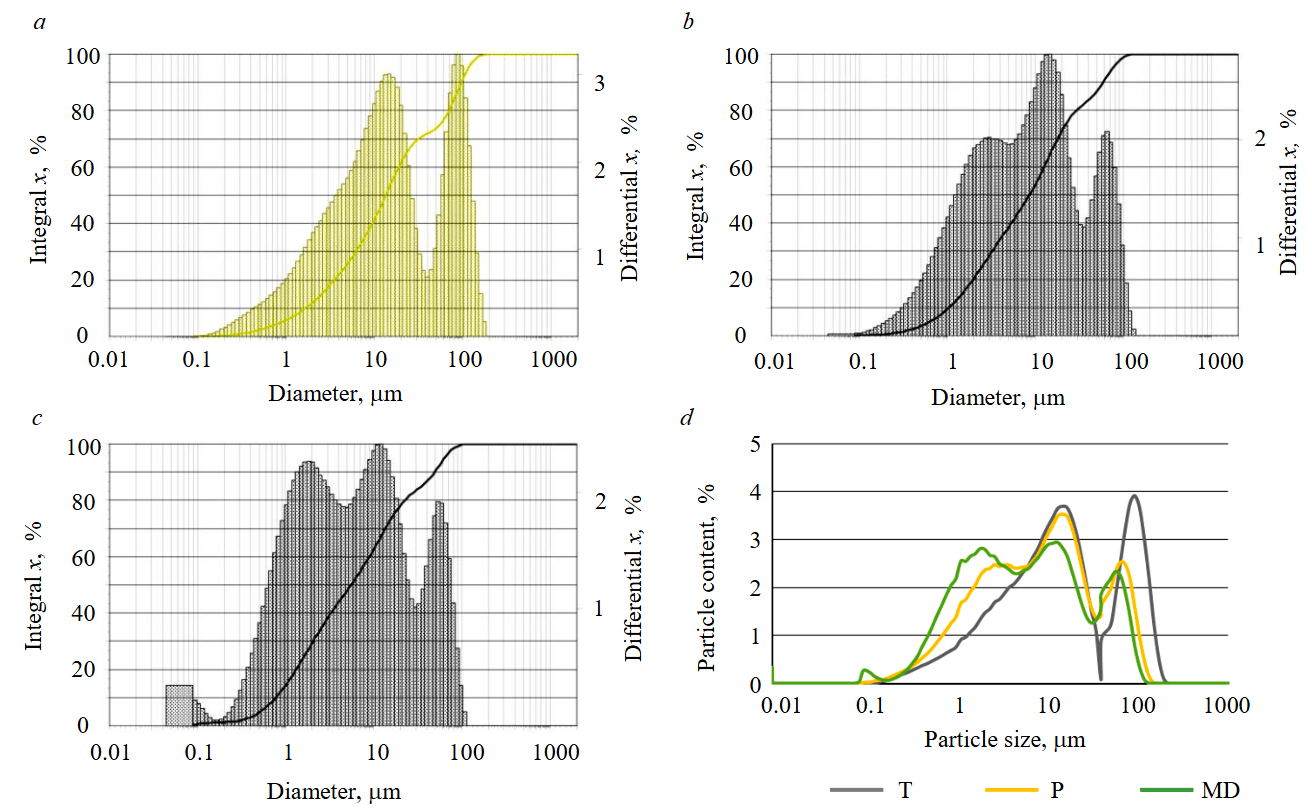
Fig.2. Particle size distribution of carbonate composition carriers: a – T; b – P; c – MD; d – comparative analysis of mineral dispersion
Based on the results of the granulometric composition determination, the ranking of carbonate materials proposed as a carrier of the photocatalytic agent was carried out according to the degree of increase in dispersion: limestone T → limestone P → marble dust.
The specific surface area of mineral materials was analyzed based on the results of the assessment of breathability (PSKh method) and nitrogen adsorption (BET method). The obtained data correlate with each other (Fig.3), however, the significant difference in values is explained by the peculiarities of studying the total specific surface area using the gas-adsorbate adsorption method, which takes into account the meso- and microporosity of the material.
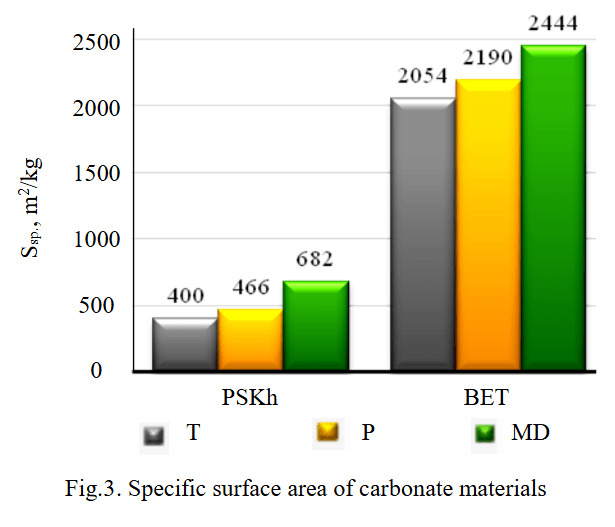
Fig.3. Specific surface area of carbonate materials
Among the studied carbonate materials, marble dust is characterized by the largest specific surface area of particles (Ssp.PSKh = 682 m2/kg, Ssp.BET = 2444 m2/kg), the smallest is the limestone of the Tyushevskoye deposit (Ssp.PSKh = 400 m2/kg, Ssp.BET = 2054 m2/kg). According to the increase in specific surface area, the materials under consideration can be ranked in the sequence: limestone T → limestone P → marble dust.
The quality of the photocatalyst’s fixation on the carrier is influenced by both chemical and physical-mechanical processes, which include imperfections of its surface – developed morphology and high porosity. During sol-gel deposition of TiO2, it is necessary to take into account the carrier porosity, the higher it is, the more photocatalytic agent can be embedded in the surface structure of the carrier material and the higher its operational efficiency will be. The use of the most dispersed raw materials with high specific surface area and porosity will lead to an increase in the active specific surface area of TiO2 in the photocatalytic composite material. The nanoporosity characteristics of the studied carbonate materials were also determined by the method of low-temperature nitrogen adsorption/desorption.
Analysis of the carbonate raw materials porosity showed the predominance of mesopores on their surface (from 2 to 50 nm according to IUPAC recommendations), the size of which ranges from 2 to 20 nm. Since limestone is a chemogenic sedimentary rock, the presence of nanometer-sized pores is characteristic of this type of material. The pore size on the surface of the studied limestone species is in the range of 2-6 nm (Fig.4, a, b). The difference in porosity of the studied raw powders is insignificant or absent, therefore, the effect on the differences in the specific surface area of these materials lies in their dispersion.
The composition and morphostructural features of the carrier largely determine the properties of its surface, which have a significant effect on the result of sol-gel synthesis and the photocatalytic activity of the composite material. High Lewis and Bronsted acidity values, the pH of the aqueous mineral solution close to the pH of the titanium dioxide sol and the pH of the matrix of the building composite, as well as low values of the ζ-potential of the raw material can increase the efficiency of fixing the photocatalytic agent on the surface of the carbonate carrier in obtaining a stable “core – shell” system. Changes in the ζ-potential make it possible to study the process of adsorption on the minerals surface, which is important when studying the mechanism of deposition of titanium compounds during sol-gel synthesis, for which the initial value of the electrokinetic potential was determined.
The efficiency of fixing titanium dioxide particles on the surface of the mineral components of the carriers largely depends on the adsorption properties of raw materials at the interface of the “solution – solid” phases [4]. The adsorption activity of the raw material is determined by the pH and ζ-potential of the aqueous mineral solution (at a concentration of 1 g/l of the raw material) and the acid-base centers of Lewis and Bronsted, in connection with which a set of indicators determining the adsorption properties of the studied components was studied.
The rock-forming mineral of the studied materials is calcite. This polymorphic modification has an isle structure consisting of CO32- groups and Са2+ cations arranged along a rhombohedron in a unit cell and which are potential limiting for this mineral. To determine the ζ-potential of calcite, it is important to take into account that, firstly, calcite is soluble in water and Са2+ and CO32- ions, depending on pH, go into solution or settle on the surface of the mineral, secondly, carbon dioxide CO2 contained in the air can interact with the solution and affect the pH and the concentration of Ca2+, CO32- and HCO3- ions [34].
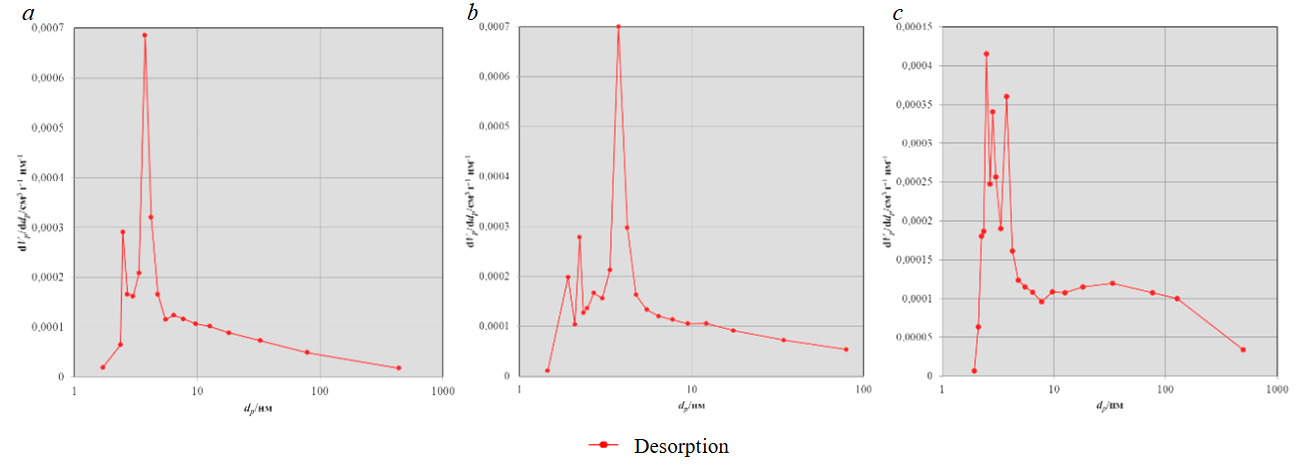
Fig.4. Porosity of carbonate materials: a – T; b – P; c – MD
The pH value for the studied carbonate materials is 10.75 for T; 10.31 for P; 9.95 for MD, which indicates their alkalinity and the presence of mainly hydroxide ions on the surface of the particles. The carbonate materials under consideration are characterized by a positive electrokinetic potential. T and P limestones are less stable with ζ-potentials of 4.78 and 4.83 mV, and marble dust, with a ζ-potential of 20.53 mV, is more stable in an aqueous mineral solution.
The formation of amorphous titanium dioxide particles in the process of sol-gel technology involves the hydrolysis and polycondensation reactions of the titanium precursor in a dispersion medium with the formation of OH-groups, and therefore an additional source of protons capable of binding titanium oxyhydrates to the surface of the carrier is an additional factor in the adhesion of titanium formations to the surface of the carrier. The formed water participates in hydrolysis, thereby condensation of the partial hydrolysis products with the oxygen-titanium chain formation. It is important to study the set of acid-base centers of Lewis and Bronsted carrier materials, which best reflects the reactivity of the surface in donor-acceptor interactions. The indicator adsorption method makes it possible to quantify the Lewis and Bronsted adsorption centers, differentiating them by type and concentration depending on the dissociation constant pKa of the indicator used [35, 36].
The distribution of acid-base active centers according to the studied indicators is similar for all studied carbonate materials (Fig.5), but there are differences in their concentration (Table 2).
The surface of limestone T demonstrated the adsorption of indicators with pKa equal to 2.5 and 10.5, which correspond to acidic and basic Bronsted centers. The concentration of the main centers of this material exceeds the concentration of acidic ones according to the set of indicators used.
Limestone P is characterized by a surface with a high concentration of active centers. With pKa equal to –0.29 (Lewis base), 2.5 (Bronsted acid), 10.5 (Bronsted base), the concentration of active centers is 52.2, 6.5 and 4.8 mmol/g. The surface of this limestone is also characterized by a higher concentration of major centers.
Table 2
Concentration of acid-base active centers on the surface of carbonate materials, mmol/g
|
Material |
Lewis bases–4.4-0 |
Bronsted acids0-7 |
Bronsted bases7-13 |
Total |
|
Limestone T |
0.66 |
7.06 |
13.87 |
21.6 |
|
Limestone P |
56.11 |
13.01 |
6.47 |
75.7 |
|
Marble dust MD |
12.37 |
28.75 |
16.4 |
57.5 |
The concentration of acid-base centers of the marble dust surface is 9.5 mmol/g at pKa = –0.29, 22.4 mmol/g at pKa = 2.5, 7.5 mmol/g at pKa = 10.5. Marble dust has a higher surface acidity than limestone, while there is also a significant content of the main centers. Marble dust has a metamorphic origin, a cleaner chemical composition and a higher specific surface area, which determines the difference in the concentration of acid-base centers from the studied limestones [4].
The highest concentrations of the main Lewis centers and the total concentration of acid-base centers according to a set of indicators are observed on the surface of limestone T (75.7 mmol/g), the highest concentration of Bronsted centers, both acidic and basic, on the surface of marble dust particles.
The acid-base properties of the carrier surface make it possible to predict the possibility and nature of sedimentation of a photocatalytic agent on it. Since hydrolysis and polycondensation reactions during sol-gel synthesis of titanium dioxide particles are accompanied by the release and addition of OH – groups, the activity of acidic Bronsted centers on the surface of carbonate materials should be taken into account for effective deposition [4]. Based on this, marble dust with a total number of Bronsted centers of 45.15 mmol/g is the most suitable for use as a carrier in a photocatalytic composite material.
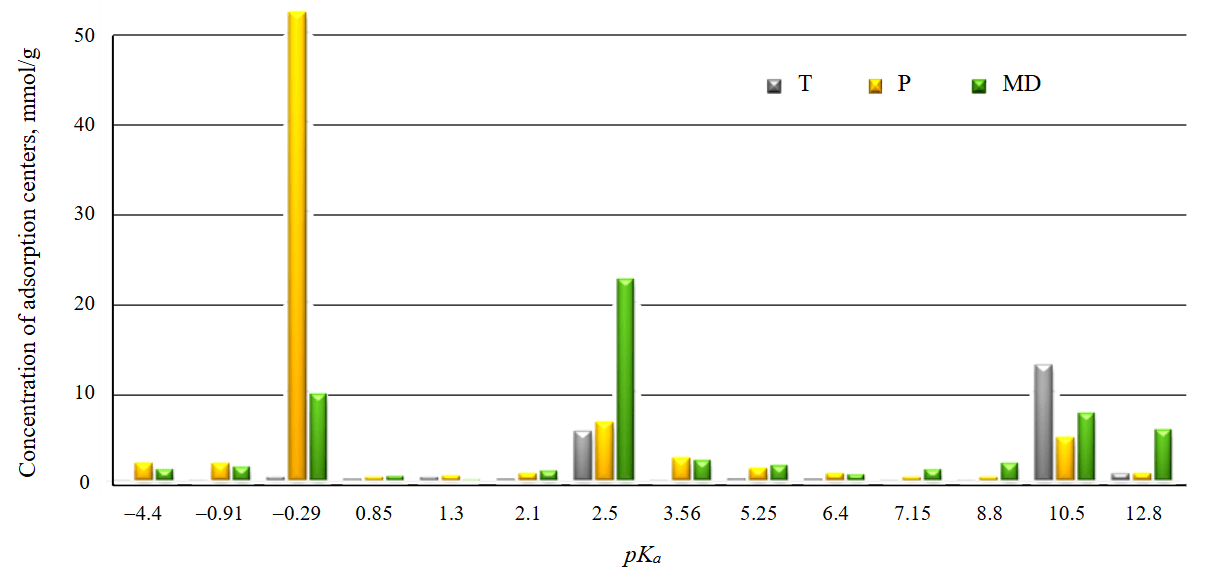
Fig.5. Distribution of acid-base centers on the surface of carbonate materials
Next, the microstructure of carbonate materials was evaluated (Fig.6), selected as the carrier of the photocatalyst composite material.
The structure of sedimentary raw materials is a collection of irregularly shaped particles of various sizes with no visible pores. Microphotographs of limestone T allow to characterize it as a powdered material with irregularly shaped particles ranging in size from 1 to 40 μm (Fig.6, a, d). Limestone P is characterized by smaller particles from 0.8 to 20 μm (Fig.6, b, e). The analysis of microphotographs of carbonate materials, which makes it possible to estimate the particle size of the material, correlates with the results of particle size distribution obtained using laser diffraction (see Fig.2).
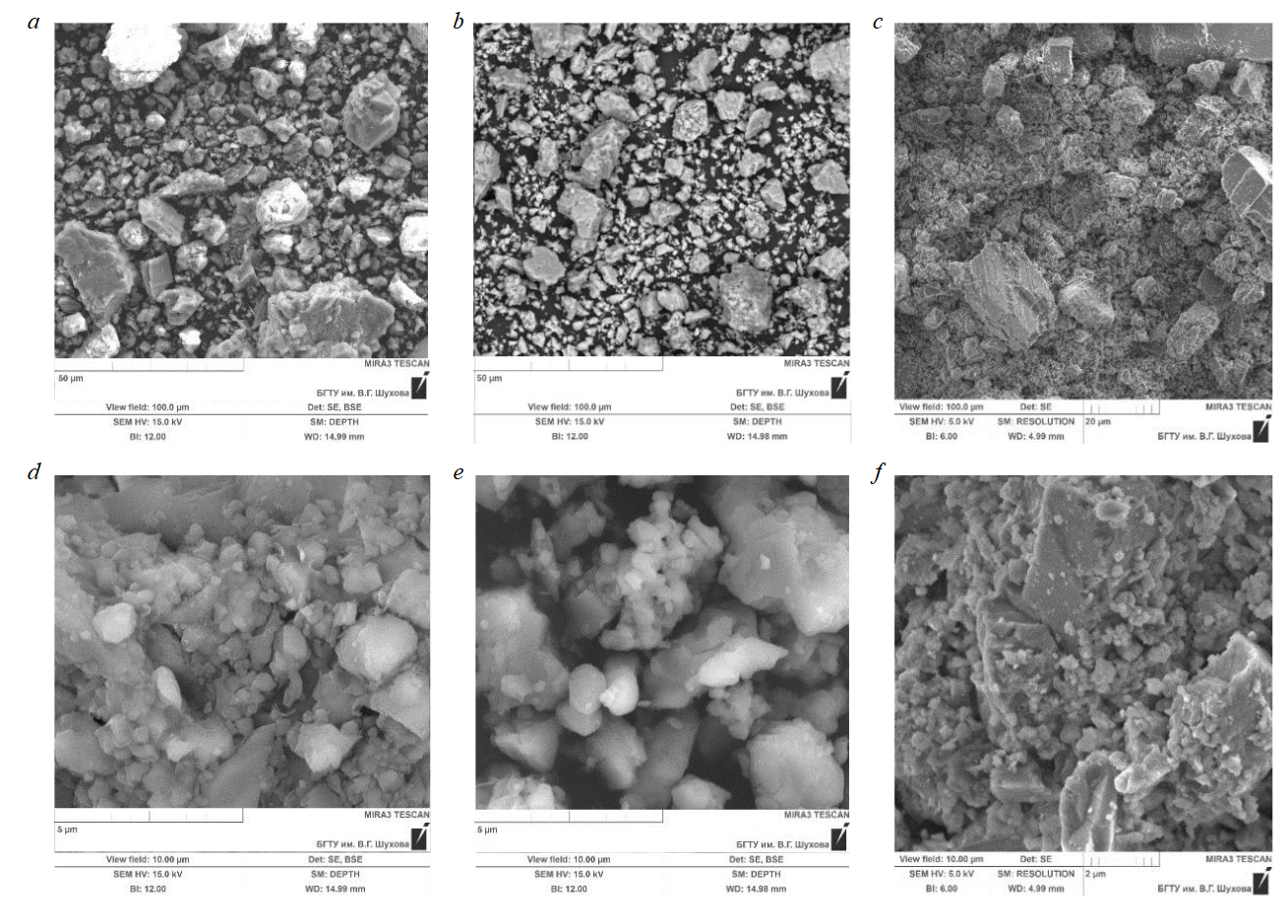
Fig.6. Morphostructural features of carbonate materials: T (a, d); P (b, e); MD (c, f)
Marble dust, due to its genesis (industrial waste), is characterized by a polydisperse distribution of flour particles ranging in size from 300 nm to 3 μm, as well as the content of larger particles of material fragments reaching 20-30 μm (Fig.6, c, f).
A comparative assessment of the physical and chemical properties of carbonate materials has been carried out and a ranking matrix has been presented in terms of the potential effectiveness of using a photocatalytic agent as a carrier in a composite material intended for use in building materials based on cement binders (Table 3).
The results of the analysis of the physical-chemical properties of the studied carbonate materials of various types indicate their compliance with the requirements for carriers in the composition of a composite material of the “carrier – photocatalyst” type for use in building materials based on cement binders. According to the set of properties, carbonate materials can be pre-ranked to increase the efficiency of their use as a carrier in the following sequence: limestone T → limestone P → marble dust MD.
To verify the preliminary ranking of carbonate raw materials according to their physical-chemical properties, a comparison was carried out with the results of assessing the photocatalytic activity of photocatalytic composite materials synthesized on their basis. The study of the possibility of using a carbonate carrier in the composition of a composite material establishes the ability of the synthesized material to organic dyes degradation. A comparison of the results of evaluating the photocatalytic activity of synthesized PCM obtained by sol-gel deposition of titanium oxides and hydroxides on a carrier followed by heat treatment at 550 °C was carried out with the widely used photocatalytic product Degussa P-25 (P-25) as a control sample.
Table 3
The ranking matrix of carbonate materials
|
Characteristic |
The sequence of increasing the potential efficiency of use |
||
| ⇒ | |||
|
Chemical composition of the raw material |
|
|
|
|
CaO content, wt.% |
Limestone T |
Limestone P |
Marble dust MD |
|
Dispersion by laser diffraction method |
Limestone T |
Limestone P |
Marble dust MD |
|
Specific surface area (PSKh) |
Limestone T |
Limestone P |
Marble dust MD |
|
Specific surface area (BET) |
Limestone T |
Limestone P |
Marble dust MD |
|
Active centers, mmol/g |
|
|
|
|
Total amount |
Limestone T |
Marble dust MD |
Limestone P |
|
Bronsted centers |
Limestone P |
Limestone T |
Marble dust MD |
An analysis of the comparison results (Fig.7) showed that the raw materials do not have photocatalytic properties. A slight decrease in the color saturation of the contaminant rhodamine B after 4 h (3-14 %) and 26 h (about 40 %) of ultraviolet light exposure is explained by the natural sorption of the dye into the volume of the powder material.
The high photocatalytic activity of the synthesized materials, exceeding that of the control sample P-25 with a lower TiO2 content in the studied samples, may be due to the specifics of the interaction of anatase with a carbonate substrate, which promotes the separation of photoexcited electrons and holes, while a high specific surface area combined with a suitable pore size can promote the adsorption of organic molecules on the surface of photocatalysts and near it [37].
Marble dust-based PCM exhibits the highest photocatalytic activity. This may be due to both its increased dispersion and the increased content of active Bronsted centers (45.15 mmol/g) acting as centers of OH– groups, which could contribute to better fixation of titanium compounds on its surface during sol-gel synthesis.
The concentrations of active Bronsted centers on the surface of T and P limestones, which contribute to the fixation of titanium dioxide particles, are close (19.48 and 20.93 mmol/g), and therefore, PCM based on them after 26 h of irradiation shows a degradation of the dye 91-93 %, which is less than that of PCM-MD (96 %), but higher than that of the control P-25 (89 %).
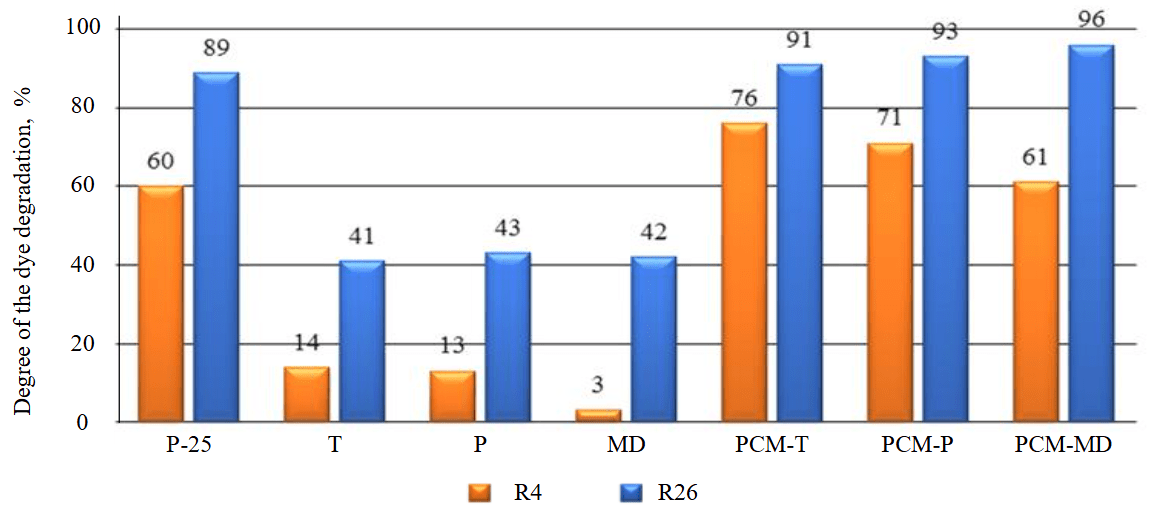
Fig.7. Discoloration of the surface of the studied materials. P-25 – photocatalytic product Degussa P-25; T and P – limestones; MD – marble dust; PCM-Т, PCM-P, PCM-MD – composite materials on appropriate carriers; R4, R26 – the time of irradiation of samples with ultraviolet light (4 and 26 h)
According to the degree of increase in photocatalytic activity, composite materials after exposure to UV light are arranged in the following order: PCM-MD – 61 %, PCM-P – 71 %, PCM-T – 76 % (4 h), PCM-T – 91 %, PCM-P – 93 %, PCM-MD – 96 % (26 h). Marble dust showed lower sorption of organic dye after 4 h of UV irradiation (3 %), which could also affect the relatively low photocatalytic activity of PCM-MD after 4 h compared with PCM on limestones.
Analysis of the results of discoloration of the contaminant showed that the photocatalyst-carrier composite materials synthesized by sol-gel deposition, where carbonate material is used as a carrier of the photocatalytic agent, are active photocatalytic systems providing oxidation-reduction reactions under the influence of ultraviolet irradiation, which leads to degradation of the contaminant.
Based on the results of the photocatalytic activity of the synthesized composite materials, it is possible to establish requirements for carbonate raw materials-carriers of the photocatalytic agent: CaCO3 content of at least 90 %, carrier dispersion in the range of 0.1-150 μm with polymodal particle distribution, specific surface area of 400-700 m2/kg, the presence of mesopores 2-50 μm in size, high activity rates of centers Lewis and Bronsted adsorption on the surface of the carrier.
The ranking according to the degree of effectiveness of carbonate raw materials is preliminary and will be clarified at the stages of sol-gel deposition, heat treatment, its physical-chemical interaction with the components of cement and concrete systems, their influence on the processes of their phase and structure formation, properties, fixation in the concrete matrix, assessment of the photocatalytic activity of concrete with PCM. The ranking of the effectiveness of carriers at various technological stages of PCM production and their comparison is necessary to establish the needed and sufficient criteria for evaluating raw materials and their weight. In the future, with the expansion of the range of raw materials used as carriers of the photocatalytic agent, this will allow for their preliminary predictive rapid assessment.
Conclusion
Waste from mining and mining processing industries are the most common types of waste. A promising way to use the host and overburden rocks located in landfills, as well as highly dispersed waste from the processing of mineral resources, is to include them in composite materials. In order to reduce the volume of waste from the main production and obtain marketable products, the possibility of using carbonate mineral materials in composites intended for incorporation into the materials and products of the construction industry is being considered. The proposed method of disposal of highly dispersed subsurface use waste will reduce the ecological burden on the environment.
The paper shows the fundamental possibility of using carbonate raw materials as a promising carrier of a photocatalytic agent in the composition of photocatalytic composite materials, which will make it possible to apply the results obtained when adapting the technology of sol-gel synthesis of PCM when using carbonate waste from various industries. Limestone powders from the Tyushevskoye (limestone T) and Porechenskoye (limestone P) deposits, as well as marble dust from the Polotskoye deposit, used in the production of building materials as mineral dispersed fillers, are considered as model systems of carbonate composition.
The physical-chemical characteristics of carbonate raw materials are determined, which are criteria for evaluating the efficiency of distribution and fixation of a photocatalytic agent during its synthesis. The particle size of the studied materials is in the range of 0.1-150 μm, specific surface area is 400-600 m2/kg, pores are 2-50 nm in size, pH is 7-11, high concentration of acid-base centers of Bronsted and Lewis, developed surface morphology. According to the set of characteristics, carbonate materials are ranked according to the increase in the potential efficiency of their use as a carrier of a photocatalytic agent in the following sequence: limestone T → limestone P → marble dust MD.
The ranking is confirmed by the results of the assessment of photocatalytic activity based on photocolorimetric measurements of the degree of degradation of the model organic contaminant rhodamine B under ultraviolet exposure to the surfaces of raw materials in their initial state and photocatalytic composite materials synthesized on their basis. It has been established that photocatalytic properties not inherent in the raw materials are actively manifested in composites synthesized on their basis and exceed the values demonstrated by the commercial photocatalyst P-25. The most photocatalytic activity (96 %) was shown by a composite material based on marble dust, for limestones T and P it was 91 and 93 %.
Thus, the fundamental possibility of using carriers of the carbonate composition in the production of photocatalytic composite materials of the “core – shell” composition is shown.
References
- Golik V.I., Mitsik M.F., Aleksakhina Yu.V. et al. Comprehensive Recovery of Metals in Tailings Utilization with Mechanochemical Activation. Resources. 2023. Vol. 12. Iss. 10. N 113. DOI: 10.3390/resources12100113
- Abbadi A., Mucsi G. A review on complex utilization of mine tailings: Recovery of rare earth elements and residue valorization. Journal of Environmental Chemical Engineering. 2024. Vol. 12. Iss. 3. № 113118. DOI: 10.1016/j.jece.2024.113118
- Krylov D.P., Klimova E.V. Origin of carbonate-silicate rocks of the Porya Guba (the Lapland-Kolvitsa Granulite Belt) revealed by stable isotope analysis (δ18O, δ13C). Journal of Mining Institute. 2024. Vol. 265, p. 3-15.
- Nerovnaya S.V. Photocatalytic composite materials and plaster mixtures using them: Avtoref. dis. ... kand. tekhn. nauk. Belgorod: Belgorodskii gosudarstvennyi tekhnologicheskii universitet im. V.G.Shukhova, 2024, p. 19 (in Russian).
- Kiyko P.I., Chernykh T.N., Sozykin S.A., Ilina L.V. About methods for measuring the efficiency of the SA-cleaning process in photo-catalytically active building materials. Expert: theory and practice. 2023. N 3 (22), р. 86-92 (in Russian). DOI: 10.51608/26867818_2023_3_86
- Tyukavkina V.V., Tsyryatyeva A.V. Fine-grained photocatalytic concretes based on titanosilicate waste. Transactions of the Kоla Science Centre of RAS. Series: Engineering Sciences. 2023. Vol. 14. N 4, p. 207-212. DOI: 10.37614/2949-1215.2023.14.4.035
- Cerro-Prada E., Torres Costa V., Manso Silván M. Sol-gel TiO2 nanoparticles prompt photocatalytic cement for pollution degradation. Advanced Material Science. 2016. Vol. 1. Iss. 1, p. 1-3. DOI: 10.15761/AMS.1000101
- Belikov M.L., Sedneva T.A., Lokshin E.P. Adsorptive and Photocatalytic Properties of Tungsten-Modified Titanium Dioxide. Inorganic Materials. 2021. Vol. 57. N 2, p. 146-153. DOI: 10.1134/S0020168521020023
- Avdin V.V., Bulanova A.V., Urzhumova A.V. Photocatalytic activity of Granular Composite TiO2/SiO2 Oxides in Destruction Reactions of Dyes. Bulletin of the South Ural State University. Series Chemistry. 2022. Vol. 14. N 2, p. 135-142 (in Russian). DOI: 10.14529/chem220214
- Chirkunova N.V., Dorogov M.V., Romanov A.E. Co-doping of titanium dioxide for photocatalysis. Technical Physics Letters. 2023. Vol. 49. N 6, p. 5-7. DOI: 10.61011/TPL.2023.06.56368.19522
- Ansari M.A., Shariq M., Ansari S.S., Husain A. Efficiency Assessment of TiO2-Based Photocatalytic Concrete for Clean and Sustainable Construction: A State-of-the-Art Review. Iranian Journal of Science and Technology, Transactions of Civil Engineering. 2024. Vol. 48. Iss. 6, p. 3871-3898. DOI: 10.1007/s40996-024-01415-8
- Fedoseev S.V., Sanneris G., Tochilo M.V. А analysis and classification of re-source saving technologies for reproduction of mineral resources of titanium industry. Journal of Mining Institute. 2016. Vol. 221, р. 756-760. DOI: 10.18454/PMI.2016.5.756
- Pathak S.S., Vesmawala G.R. Influence of Nano-TiO2 and water to cement ratio on fracture parameters of concrete. Asian Journal of Civil Engineering. 2023. Vol. 24. Iss. 7, p. 1969-1979. DOI: 10.1007/s42107-023-00616-2
- Batista G. dos S., Takimi A.S., da Costa E.M. Hardened oil well cement paste modified with TiO2@SiO2 nanoparticles: Physical and chemical properties. Construction and Building Materials. 2023. Vol. 367. N 130282. DOI: 10.1016/j.conbuildmat.2022.130282
- Zengkun Li, Haiyan He, Xuan Wang et al. Robust SiO2@TiO2 nanocoatings with antireflection and photocatalytic self-cleaning properties by introducing commercial P25 TiO2. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2023. Vol. 664. N 131176. DOI: 10.1016/j.colsurfa.2023.131176
- Yadav M., Arora R., Dhanda M. et al. Ppy/TiO2-SiO2 nanohybrid series: synthesis, characterization, photocatalytic activity, and antimicrobial potentiality. Journal of Environmental Health Science and Engineering. 2023. Vol. 21. Iss. 1, p. 239-254. DOI: 10.1007/s40201-023-00858-x
- Singh K., Kumar A., Singh A.K., Agarwal A. Fly ash and TiO2 modified fly ash as adsorbing materials for effective removal of methylene blue and malachite green from aqueous solutions. Journal of the Indian Chemical Society. 2023. Vol. 100. Iss. 3. N 100942. DOI: 10.1016/j.jics.2023.100942
- Visa M., Cosnita M., Moldovan M. et al. Fly Ash Waste Recycling by Pt/TiO2 Incorporation for Industrial Dye Removal. International Journal of Environmental Research and Public Health. 2021. Vol. 18. Iss. 8. N 3887. DOI: 10.3390/ijerph18083887
- Petcu G., Papa F., Anghel E.M. et al. Effects of Aluminosilicate Gel Treatment and TiO2 Loading on Photocatalytic Properties of Au-TiO2/Zeolite Y. Gels. 2023. Vol. 9. Iss. 6. N 503. DOI: 10.3390/gels9060503
- Wen Cui, Jieyuan Li, Lvcun Chen et al. Nature-inspired CaCO3 loading TiO2 composites for efficient and durable photocatalytic mineralization of gaseous toluene. Science Bulletin. 2020. Vol. 65. Iss. 19, p. 1626-1634. DOI: 10.1016/j.scib.2020.05.024
- Samchenko S.V., Alexandrova O.V., Gurkin A.Yu. Properties of cement composites based on limestone depending on their granulometric composition. Vestnik MGSU. 2020. Vol. 15. Iss. 7, p. 999-1006 (in Russian). DOI: 10.22227/1997-0935.2020.7.999-1006
- Turemuratov Sh., Najimova N. Сhemical and physico-chemical character of carbonic minerals at Ustyurt. Universum: khimiya i biologiya. 2020. N 10-1 (76), p. 61-63 (in Russian).
- Lozovaya S.Yu., Chentsov A.E., Sevostyanov A.E. Analysis of the application of carbonate rocks in construction. Ehnergosberegayushchie tekhnologicheskie kompleksy i oborudovanie dlya proizvodstva stroitelnykh materialov: Mezhvuzovskii sbornik statei. Belgorod: Belgorodskii gosudarstvennyi tekhnologicheskii universitet im. V.G.Shukhova, 2018. Iss. XVII, p. 214-217 (in Russian).
- Ahmad I., Shen D., Khan K.A. et al. Effectiveness of Limestone Powder in Controlling the Shrinkage Behavior of Cement Based System: a Review. Silicon. 2022. Vol. 14. Iss. 2, p. 359-371. DOI: 10.1007/s12633-020-00897-1
- Belov V.V., Kuliaev P.V., Barkaya T.R. Mechanical properties of fine-grained carbonate concretes with a complex additive, including fine limestone filler and superplasticizer. Structural Mechanics of Engineering Constructions and Buildings. 2023. Vol. 19. N 2, p. 251-257 (in Russian). DOI: 10.22363/1815-5235-2023-19-2-251-257
- Tarakanov O.V., Belyakova E.A., Moskvin R.N. Application of mineral sludge and carbonate rocks in the production of cement materials. Expert: theory and practice. 2023. N 1 (20), р. 130-132 (in Russian). DOI: 10.51608/26867818_2023_1_130
- Strokova V.V., Gubareva E.N., Ogurtsova Yu.N. Evaluation of the properties of the silica raw materials as a substrate as component of composite photocatalytic material. Bulletin of Belgorod State Technological University named after V.G.Shukhov. 2017. N 2, p. 6-12 (in Russian).
- Strokova V., Gubareva E., Ogurtsova Y. et al. Obtaining and Properties of a Photocatalytic Composite Material of the “SiO2-TiO2” System Based on Various Types of Silica Raw Materials. Nanomaterials. 2021. Vol. 11. Iss. 4. N 866. DOI: 10.3390/nano11040866
- Gang Liao, Wu Yao, Anming She et al. Interfacial design of nano-TiO2 modified recycled concrete powder for building self-cleaning. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2023. Vol. 661. N 130925. DOI: 10.1016/j.colsurfa.2023.130925
- Suleymanova L.A., Maliukova M.V., Ryabchevskiy I.S. et al. Illuminated concrete using rock crushing waste. Bulletin of Belgorod State Technological University named after V.G.Shukhov. 2020. N 12, p. 8-16. DOI: 10.34031/2071-7318-2020-5-12-8-16
- Korchunov I.V., Potapova E.N., Sivkov S.P. et al. Limestone in cement compositions with additives to increase frost resistance. Cement and its Applications. 2022. N 2, p. 44-49 (in Russian).
- Rakhimbaev Sh.M., Tolypina N.M., Kosinova A.A., Khakhaleva E.N. Influence of Electro-Surface Properties of Mineral Filler on Frost Resistance of Powder Concretes. Stroitelnye Materialy. 2019. N 10, p. 12-15 (in Russian). DOI: 10.31659/0585-430X-2019-775-10-12-15
- Kolmogorov A.Y., Korchunov I.V., Potapova E.N. The effect of mineral additives on the frost resistance of hardened cement. Uspekhi v khimii i khimicheskoi tekhnologii. 2022. Vol. XXXVI. N 3, p. 83-85 (in Russian).
- Anashkina N.E. Experimental justification of the mechanism of modification of physico-chemical, structural and technological properties of diamonds and rock-forming minerals of kimberlites under non-thermal action of high-voltage nanosecond pulses: Avtoref. dis. ... kand. tekhn. nauk. Moscow: Institut problem kompleksnogo osvoeniya nedr im. akademika N.V.Melnikova, 2019, p. 26 (in Russian).
- Petrunin M.A., Maksayeva L.B., Yurasova T.A. The role of acid-base interactions in metal corrosion. Review. Korroziya: zashchita materialov i metody issledovanii. 2024. N 3, p. 1-43 (in Russian). DOI: 10.61852/2949-3412-2024-2-3-1-43
- Skvortsova L.N., Tikhonova I.A., Dychko K.A. et al. Acid-Base Properties and Adsorption Activity of Iron-Containing Composites in the Photocatalytic Degradation of Organic Pollutants. Russian Journal of Physical Chemistry A. 2024. Vol. 98. N 10, p. 2202-2210. DOI: 10.1134/S0036024424701346
- Hai D. Tran, Dinh Quan Nguyen, Phuong T. Do, Uyen N.P. Tran. Kinetics of photocatalytic degradation of organic compounds: a mini-review and new approach. RSC Advances. 2023. Vol. 13. Iss. 25, p. 16915-16925. DOI: 10.1039/D3RA01970E
