Efficiency of acid sulphate soils reclamation in coal mining areas
- 1 — Ph.D. Senior Researcher Natural Science Institute, Perm State National Research University ▪ Orcid
- 2 — Ph.D., Dr.Sci. Leading Researcher Natural Science Institute, Perm State National Research University ▪ Orcid
- 3 — Ph.D. Leading Researcher Natural Science Institute, Perm State National Research University ▪ Orcid
- 4 — Junior Researcher Natural Science Institute, Perm State National Research University ▪ Orcid
Abstract
During the development of coal deposits, acid mine waters flowing to the surface cause the formation of acid sulphate soils. We study the effectiveness of soil reclamation by agrochemical and geochemical methods at the site of acid mine water discharge in the Kizel Coal Basin, carried out in 2005 using alkaline waste from soda production and activated sludge. A technosol with a stable phytocenosis was detected on the reclaimed site, and soddy-podzolic soil buried under the technogenic soil layer with no vegetation on the non-reclaimed site. The buried soddy-podzolic soil retains a strong acid рН concentration Н2О = 3. A high content of organic matter (8-1.5 %) is caused by carbonaceous particles; the presence of sulphide minerals reaches a depth of 40 cm. Technosol has a slightly acid pH reaction H2O = 5.5, the content of organic matter due to the use of activated sludge is 19-65 %, the presence of sulphide minerals reaches a depth of 20-40 cm. The total iron content in the upper layers of the technosol did not change (190-200 g/kg), the excess over the background reaches 15 times. There is no contamination with heavy metals and trace elements, single elevated concentrations of Li, Se, B and V are found.
Introduction
Coal production is the leading factor in the transformation of the natural environment in the areas of coal mining [1-3]. Shaft coal mining is usually accompanied by pumping and discharge of mine waters, as well as the accumulation of large-tonnage overburden dumps [4, 5]. Coals and coal-bearing rocks contain a large amount of sulphide and organic sulphur. For example, the content of pyrite in overburden can reach 10-12 % [6]. Pyrite and other sulphides in the oxidizing conditions of the mine space and rock dumps are chemically unstable under the influence of water and the participation of microorganisms [7, 8], so acid waters enriched with sulphates are formed in the mine space and rock dumps.
Discharge of mine waters to the surface and formation of acid wastewater of rock dumps, as well as the transfer of particles of rock dumps by temporary water flows leads to technogenic impact on the soils in adjacent areas [9-11]. Wastewater from dumps and spills of mine waters are sources of trace elements entering surface waters, bottom sediments, and soils [4, 10, 12]. As a result, technogenic soils are formed with physical and chemical properties that are not characteristic of the original soils [9], with a strong acid reaction of the environment and an increased content of sulphates and trace elements in the surface layer [9, 12, 13]. Retention capacity of soils provides the possibility of secondary contamination of surface and ground waters with trace elements and sulphates [14].
Reclamation of dumps and disturbed areas, remediation of soils and higher vegetation is an important applied task in the remediation of technogenic ecosystems [15, 16].
Soil formation in mining areas depends on the climate and topography, chemical composition of the dump rocks, which are technogenic soil-forming rock [17, 18]. Acid sulphate soils occur in coal mining areas due to the inflow of mine and waste water enriched with sulphates. Overburden dumps are an additional source of organic matter. In natural conditions, the formation of acid sulphate soils is confined to substrates rich in sulphides. For example, sulphidization manifests itself as a result of the penetration of sea water into tidal marshes with anaerobic conditions and a high content of organic matter [19, 20].
The study of technogenic soil formation and the effectiveness of reclamation measures was carried out in the Kizel coal deposit (Perm Krai). Despite the detailed study of surface waters and bottom sediments [4, 21, 22], the current state of soils in self-spills and acid mine waters discharge sites in the Kizel Coal Basin (KCB) has not been practically investigated. In the 20s of the XXI century, attempts were made to study soils formed on dumps [23, 24], however, the study of soils formed on runoff from dumps and mine water runoff was not carried out even before the reclamation activities.
The purpose of this work is to investigate the original and reclaimed soils at the site of acid water discharge from the Shirokovskaya mine in order to assess the effectiveness of the reclamation carried out in 2005. The tasks are to study the morphological changes in soils and their classification; investigate the chemical properties of soils, make a geochemical assessment of soils by the content of trace elements.
Methods
Description of the study area
The Kizel Coal Basin is in the eastern part of the Perm Krai, its area is 1500 km2, its length is about 150 km. Underground coal mining lasted about 200 years. From 1992 to the early 2000s, the mines were abandoned. In the KCB area, there are about 100 rock dumps. Earth remote sensing (ERS) methods established that the land area occupied by dumps is 260 ha [4]. Over 35 million m3 of rocks were accumulated in the dumps during the mine operation. The lithology of the coal-bearing stratum, the mining and storage technology determined the heterogeneity in the mineral composition of the rocks of the dumps, in which about 60 minerals were found. Dumps consist of mudstones, siltstones, sandstones, limestones, coal, pyrite, and may contain wood and metal features [25]. The long-term operation of mines and their inappropriate abandonment led to negative consequences for surface and ground waters and soils. Self-spills of mine waters to the surface and runoff from dumps have a strong acid reaction, pH 2-3, are characterized by high concentrations of iron, aluminium, manganese, beryllium, the content of which is hundreds of times higher than the maximum permissible concentration (MPC) [4, 10, 26].
The study area belongs to the Ural geochemical province of the eluvial-transalluvial region of the residual mountain ranges of the western slope of the Middle Urals, to the Upper Yaiva landscape of high ridged and steeply sloping foothills on the Paleozoic carbonate and partially terrigenous rocks. The climate is moderate continental, the average annual rainfall is 700 mm. The KCB area belongs to the Western foothill region of heavy loamy podzolic, soddy-podzolic, and marshy soils. According to the botanical and geographical zoning, the study area belongs to the middle and southern taiga foothill fir-spruce and spruce-fir forests.
The sampling area is in the Kizel district of the Perm Krai, near the dump of the Shirokovskaya mine, 0.3 km from the village of Yuzhny Kospashsky (Fig.1).
Research objects
In 2021, two soil profiles were made at the site of acid mine water discharge at an altitude of 370 m above sea level. The sampling area is 350 m from the dump of the Shirokovskaya mine: section N 1 directly at the site of acid mine waters discharge, section N 2 at the reclaimed discharge site (Fig.2). Samples of soddy-podzolic soil were taken as a background; the pit was made in a secondary small-leaved forest 30 m from the site of the former discharge.
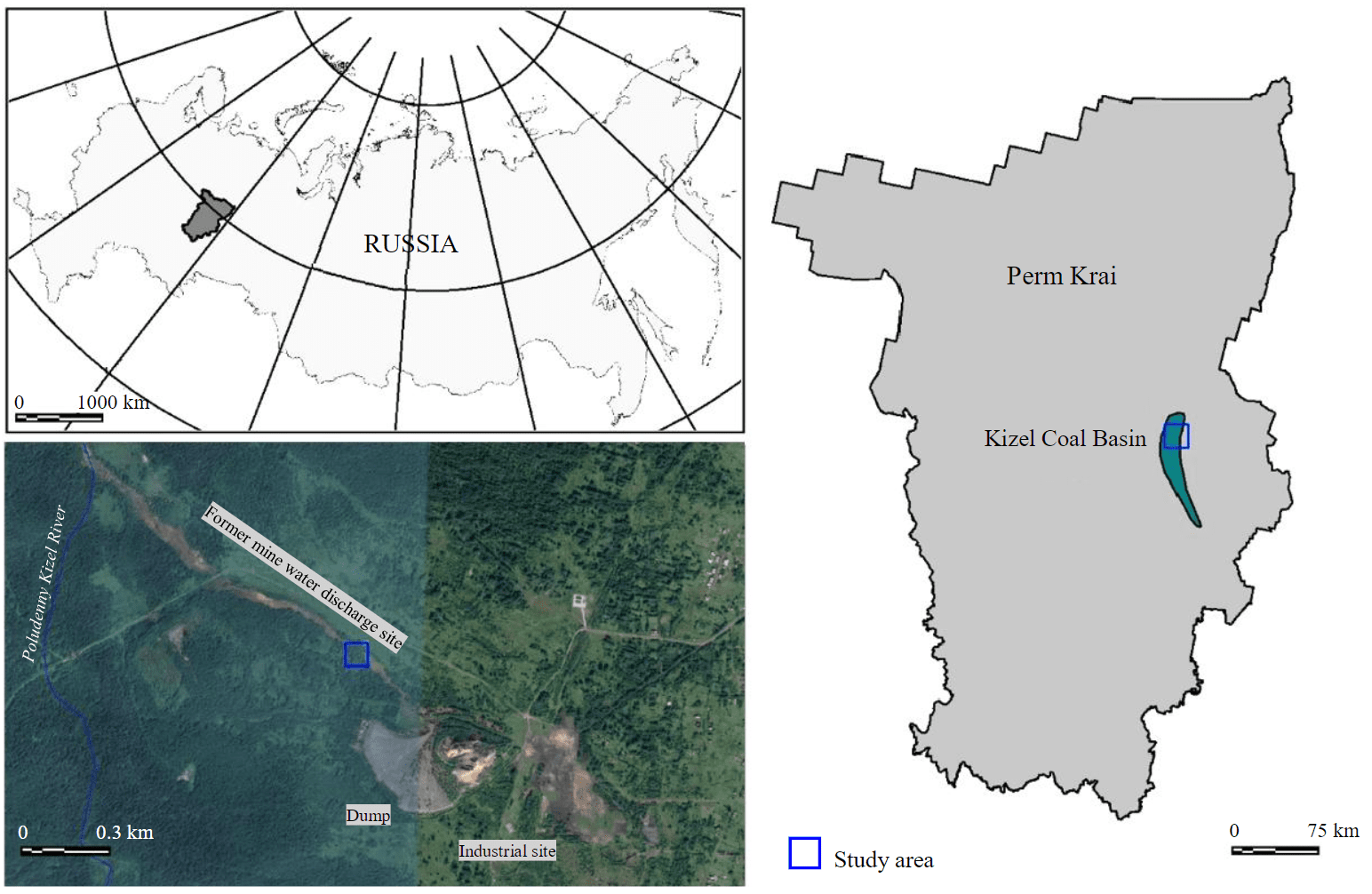
Fig.1. Geographical position of the Kizel Coal Basin
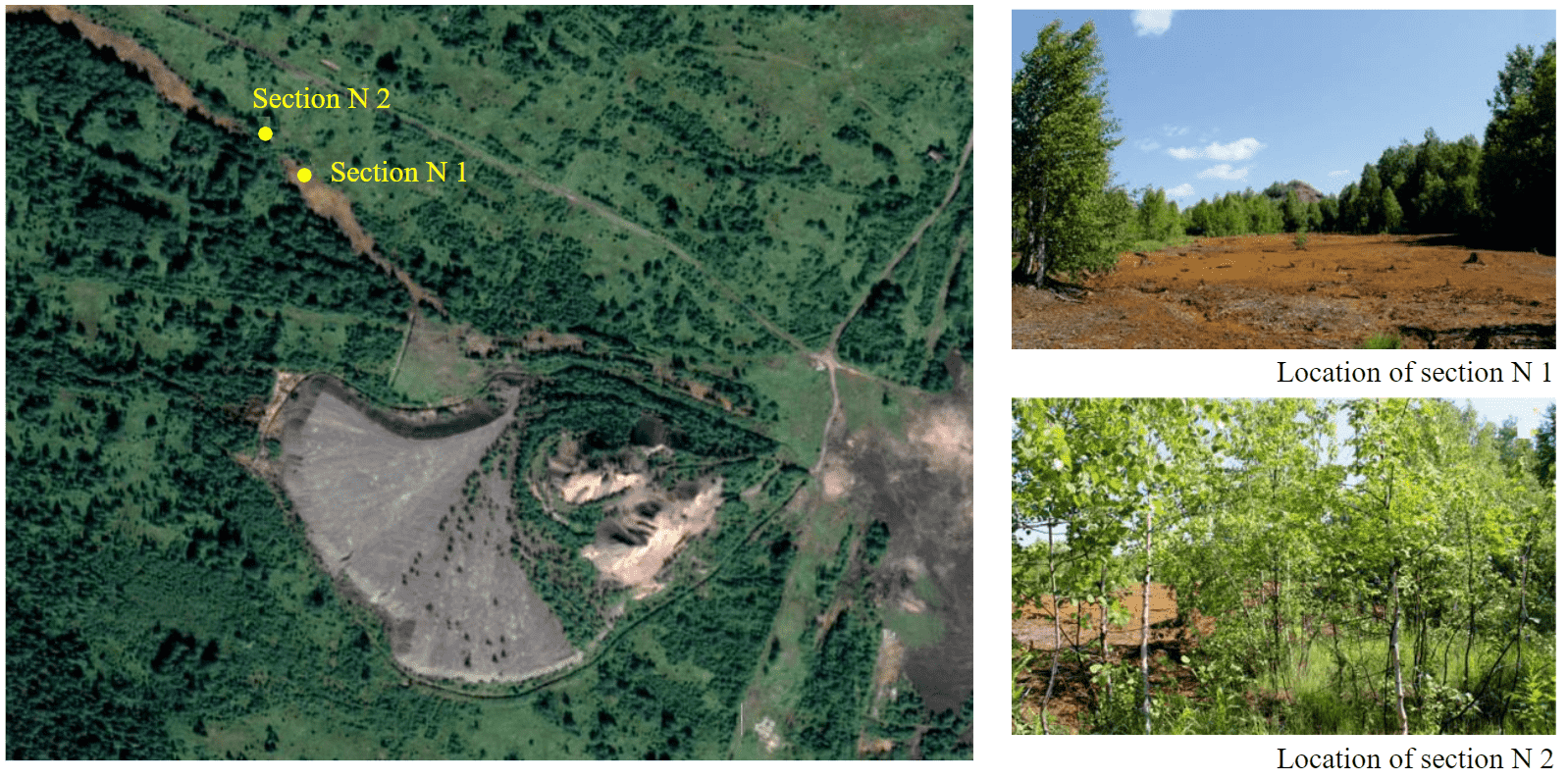
Fig.2. Sampling area
The acid mine water discharge site is a strip 10-90 m wide, extended in a north-western direction from the area of the former mine and the waste dump to the Poludenny Kizel River. The length of the site is about 1.8 km, its area in 2021, according to remote sensing data, was about 20 ha. The surface of the site has a red-brown colour and is characterized by a complete absence of vegetation. The remains of dead woody plants are noted.
The Shirokovskaya mine started its operation back in 1945, was abandoned at the end of the 1990s. Approximately in the mid-1980s, acid mine waters were neutralized with lime. As a result, a large amount of technogenic soils, consisting of finely dispersed iron hydroxides with crushed stone, grus, and sand of mine dumps, accumulated at the discharge site. The thickness of technogenic soils in some places can reach one metre.
Soil reclamation in a small area of the acid mine water discharge site near the Shirokovskaya mine dump was carried out about 17 years ago. It consisted in creating an artificial alkaline geochemi-cal barrier that helps to reduce the migration of pollutants by transferring them into immobile forms [27]. To reduce the acidity of soils, alkaline wastes from OAO Berezniki Soda Plant (BSP) were used as a reactant. They consisted of finely dispersed CaCO3 by more than 90 % and had no harmful admixtures. The activated sludge from the treatment facilities of OAO Metafrax (Gubakha) was used as organic matter [27]. The composition of the grass mixture for reclamation included reed canary grass Phalaris arundinacea L., red clover Trifolium praténse L., alfalfa Medicago sativa L., and couch grass Elytrigia repens (L). Reclamation measures on the soils with acid sulphate runoff led to a decrease in acidity from 2.7-3.0 to 5.0-6.0. Land reclamation in coal mining areas is often based on a decrease in acidity and an increase in fertility of technogenic soils in general. Reclamation includes fertilization to regulate nutrients, nitrogen, organic matter, as well as measures to improve the physical properties of soils [28]. In Europe, reclamation activities in dumps and mining areas are carried out for the emergence of sustainable tree plantations, which indicate the ecosystem restoration [1]. The introduction of lime during the reclamation of dumps is a fairly common method [28], as is the introduction of activated sludge as a fertile layer [29].
Currently, a stable phytocenosis was found on the reclaimed site, represented by young birches (genus Betula), willows (genus Salix), and alder (genus Alnus), as well as herbaceous plants of the sedge (genus Carex), graminaceous Poaceae, fireweed Onagraceae, pink Caryophyllaceae families, as well as mosses.
Sampling and research methods
In soil profiles, samples were taken to a depth of 80 cm with a step of 10 cm. Actual and exchangeable acidity was determined by the potentiometer. Determination of soil acidity in hydrogen peroxide was carried out for the oxidation of sulphide minerals present in dumps and runoff from them (R.Brinkman and L.J.Pons[1] proposed a preliminary pH limit of H2O2 = 2.5 for hazardous acid sulphate soils after peroxide treatment). Hydrolytic acidity was determined by the Kappen method (in 1M СH3COONa extract), based on titration with 1 N alkali in the presence of phenolphthalein. Exchangeable acidity (EA), exchangeable aluminium Alex and exchangeable hydrogen Nex were studied according to the Sokolov method, based on soil treatment with a 1M KCl solution, followed by titration of one part with alkali to discover the sum of exchangeable aluminium and hydrogen, the other part of the extract was titrated with alkali with adding fluoride to determine hydrogen ions. The content of organic matter was determined by spectrophotometry. The cation exchange capacity (CEC) was determined by the Bobko – Askinazi – Alyoshin method. The content of exchangeable calcium and exchangeable (mobile) magnesium was studied by complexometric titration. The content of mobile sulphur was determined by turbidimetry, mobile iron was determined by spectrophotometry with o-phenanthroline. Sulphate ions in the aqueous extract were determined by turbidimetry.
The microelement composition was determined by inductively coupled plasma spectrometry on an Elan 900 mass spectrometer, the particle size distribution was determined using a set of “Vibrotechnik” sieves and a laser diffraction particle size analyser Analysette 22 Micro Tec plus at the Centre for Unique Research Equipment of the Perm State National Research University.
To assess the level of soil contamination with chemical elements, the Igeo index of geological accumulation of elements in soils was used, which links the content of elements of the natural soil background with the influence of human activity on the content of elements in technogenically disturbed soil. The index, widely used to assess the contamination of surface soil layers with trace elements [30, 31], was calculated for each element
where Cn is the content of the microelement in the soil; Bn is the local natural background content of the microelement in the soil; k is the index of background content compensation due to lithogenic factors, usually set equal to 1.5 [30, 31].
Igeo is divided into seven classes: Igeo ≤ 0 – uncontaminated; 0 < Igeo < 1 – uncontaminated to moderately contaminated; 1 < Igeo < 2 – moderately contaminated; 2 < Igeo < 3 – moderately contaminated to highly contaminated; 3 < Igeo < 4 – highly contaminated; 4 < Igeo < 5 – highly conta-minated to very highly contaminated; 5 < Igeo – very highly contaminated.
An environmental assessment of the soil-vegetative layer (0-20 cm) according to the degree of total soil contamination with heavy metals was carried out using the contamination index Zc, calculated according to SanPin 1.2.3685-21.
Discussion of the results.
Morphology and classification of soils
Section N 1 was made on the unreclaimed site of acid mine water discharge. The soil profile consists of several layers (Fig.3, a). The upper layer is formed by technogenic material accumulated during the discharge of acid mine waters and their neutralization with lime, as well as mechanical deposition of sand and gruss of the dump rocks. Below the technogenic horizon, a profile of soddy-podzolic soil was found, consisting of humus, eluvial, transitional horizons, and horizon differentiated by texture.
The technogenic TCH layer is 0-33/33 cm, brown-orange, structureless, dense, viscous, clayey. The presence of sand and gruss of overburden rocks of the dump (2-10 mm) is noted, there are no pores, the boundary of the next layer is well defined. The volume of clastic particles is about 60 % of the soil mass.

Fig.3. Soil profiles: a – soddy-podzolic soil buried under the technogenic layer; b – technosol
Humus horizon AY is 33-35/2 cm, dark grey, finely cloddy, clayey.
Eluvial horizon EL is 35-45/10 cm, pale-yellow, rusty spots on the soil pads, clayey, moist, unstructured in the field, when dry it breaks in one plane, which indicates layering.
Subeluvial horizon BEL is 45/60/15 cm, light yellow-pale, presence of ferruginous concretions, clayey, in a dry state breaks up into prisms, dense, porous.
Horizon differentiated by texture BT is 60-80/20 cm, pale-brown in dry state, reddish-brown in wet state, rusty stains, clayey, prismatic structure found in dry state. According to the classification and diagnostics of soils in Russia, the soil is diagnosed as a soddy-podzolic clayey soil buried under a technogenic layer.
According to the WRB World reference base for soil resources, the soil is identified as Epitechno-leptic Technosols (Supraalbic, Epithionic, Clayic, Phytotoxic, Skeletic).
Profile N 2 is described on the reclaimed site. This soil has several layers of different colours (Fig.3, b). The soil is clayey, viscous, compacted. On the surface there are fallen leaves and grassy remains, below the leaves there is a technogenic horizon of brown-ochre colour, 29 cm thick, with black interlayers and reddish spots. At a depth of 20 cm, white inclusions are observed. Apparently, these are waste from soda production. From 29 to 52 cm, the colour of the profile becomes almost black, layering and lack of structure are observed. Below, to 80 cm, there is a grey-brown clayey unstructured layer. The soil was diagnosed as technosol on soddy-podzolic soil. This profile structure was formed as a result of the introduction of soda production waste and activated sludge, their mixing and migration along the profile over the next years. According to WRB, the soil is diagnosed as Technosols (Clayic, Histic, Gleyic). Both types of soils were formed under hydromorphic conditions in a trans-accumulative landscape.
The third profile is a typical soddy-podzolic clayey soil; under the humus horizon there is an eluvial horizon, then a transitional horizon, and then a horizon differentiated in texture; the presence of a thin litter is noted.
Chemical properties of soils
The soddy-podzolic soil buried under the technogenic layer to a depth of 30 cm has a strong acid reaction, pH H2O = 3.0 (Fig.4, a), acidity decreases with depth; pH H2O2 to 30 cm does not exceed 2.5, which indicates the presence of sulphide minerals. High acidity is characteristic of the technogenic layer, which is represented by crushed matter of overburden dump rocks. Oxidation of sulphide minerals and acidification of the substrate occur due to the presence of pyrite in the mineral composition of rocks [4, 25]. The buried soddy-podzolic soil is characterized by an acid reaction, the acidity of the horizons corresponds to the acidity of the corresponding horizons in the background soil.
Technosol to 20 cm has a weak acid reaction, pH H2O = 5.3-4.4; to a depth of 50 cm, the acidity sharply increases to pH H2O = 3.3 (Fig.4, b). The indicators of actual acidity correlate with the pH values of H2O2; from a depth of 30-50 cm, sulphide minerals are present in the technosol, providing a strong acid soil reaction. The decrease in acidity in the upper layers of the technosol is caused by the soil neutralization as a result of the introduction of soda production waste during the reclamation.
The hydrolytic acidity Hha of the entire profile of the soddy-podzolic soil buried under the technogenic layer and the background soil does not differ, its value is due to the presence of acid technogenic deposits and the properties of the soddy-podzolic buried soil. In the technosol, the value of hydrolytic acidity to 50 cm is lower than in the soddy-podzolic buried and background soil, which was promoted by the reclamation measures carried out (Table 1).
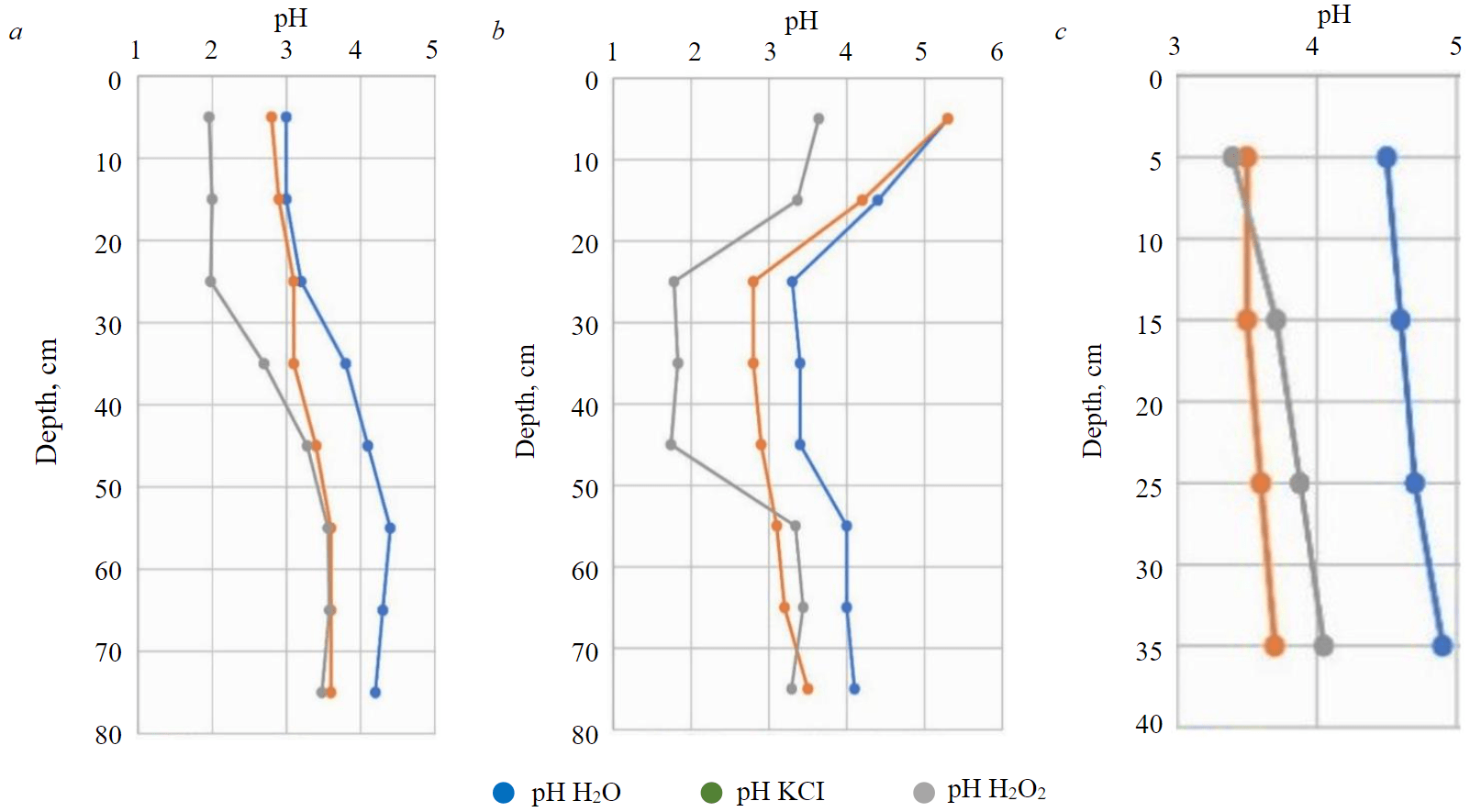
Fig.4. Soil acidity: a – soddy-podzolic, buried under the technogenic layer; b – technosol; c – background soddy-podzolic soil
Table 1
Soil chemical properties
|
Soil |
Depth, cm |
Corg, % |
Нha,mmol/100 g |
CEC,mmol/100 g |
Alex,mmol/100 g |
EA,mmol/100 g |
Hex,mmol/100 g |
SO42–,mmol/100 g |
Caex,mmol/100 g |
Femob, (total),mg/kg |
Fetot,g/kg |
Smob,mg/kg |
|
Soddy-podzolic, buried under the technogenic layer |
0-10 |
8.2 |
18.9 |
31.0 |
0.62 |
0.75 |
0.13 |
1.7 |
0.50 |
460 |
200.6 |
724 |
|
10-20 |
6.5 |
18.1 |
25.0 |
0.59 |
0.69 |
0.15 |
1.6 |
0.50 |
530 |
200.8 |
698 |
|
|
20-30 |
8.3 |
16.2 |
24.0 |
0.78 |
0.81 |
0.03 |
1.2 |
0.50 |
620 |
109.8 |
601 |
|
|
30-40 |
4.7 |
24.7 |
22.0 |
6.11 |
7.45 |
1.34 |
1.1 |
0.88 |
200 |
18.7 |
182 |
|
|
40-50 |
2.35 |
21.1 |
24.0 |
5.56 |
5.60 |
0.04 |
0.6 |
0.50 |
250 |
11.7 |
129 |
|
|
50-60 |
1.5 |
12.5 |
20.0 |
5.33 |
7.30 |
1.91 |
0.7 |
1.00 |
180 |
13.3 |
158 |
|
|
60-70 |
1.4 |
14.3 |
20.0 |
6.67 |
6.60 |
0 |
0.6 |
4.25 |
170 |
13.4 |
148 |
|
|
70-80 |
2.2 |
16.2 |
13.0 |
5.22 |
5.90 |
0.62 |
0.8 |
1.38 |
170 |
16.0 |
43 |
|
|
Technosol |
0-10 |
19.1 |
4.73 |
31.0 |
0.09 |
0.14 |
0.06 |
7.4 |
7.31 |
380 |
197.8 |
2000 |
|
10-20 |
23.0 |
8.40 |
27.0 |
0.01 |
0.04 |
0.03 |
1.9 |
2.25 |
450 |
190.1 |
869 |
|
|
20-30 |
41.4 |
14.2 |
23.0 |
1.89 |
2.00 |
0.11 |
0.9 |
1.19 |
310 |
66.3 |
331 |
|
|
30-40 |
65.8 |
11.0 |
21.0 |
2.00 |
2.17 |
0.19 |
0.9 |
1.00 |
200 |
33.1 |
79 |
|
|
40-50 |
65.8 |
13.7 |
22.0 |
2.44 |
2.51 |
0.04 |
0.7 |
1.06 |
220 |
41.9 |
349 |
|
|
50-60 |
2.51 |
17.9 |
20.0 |
3.33 |
6.20 |
2.90 |
0.9 |
0.88 |
210 |
14.1 |
142 |
|
|
60-70 |
1.4 |
15.8 |
24.0 |
6.56 |
6.60 |
0.06 |
0.8 |
0.50 |
260 |
17.3 |
117 |
|
|
70-80 |
1.1 |
18.5 |
21.0 |
4.11 |
4.70 |
0.62 |
1.1 |
0.75 |
170 |
18.6 |
159 |
|
|
Soddy-podzolic (background) |
0-10 |
5.0 |
26.4 |
13.0 |
4.78 |
5.0 |
0.17 |
0.05 |
3.25 |
430 |
13.5 |
12.9 |
|
10-20 |
5.1 |
21.4 |
21.0 |
5.78 |
5.9 |
0.12 |
0.05 |
2.25 |
430 |
13.4 |
4.6 |
|
|
20-30 |
6.1 |
20.8 |
19.0 |
5.78 |
5.9 |
0.13 |
0.05 |
2.75 |
360 |
11.6 |
0.2 |
|
|
30-40 |
2.5 |
19.9 |
17.0 |
5.56 |
6.7 |
1.12 |
1.30 |
2.75 |
260 |
13.1 |
0.9 |
The maximum content of organic matter Corg is typical for 30-50 cm of the technosol layer, 65.8 %, in general, in the technosol to 50 cm, the amount of organic matter varies from 19.1 to 65.8 % (Table 1). This is due to the introduction of activated sludge during the reclamation and its migration along the profile over a 15-year period. The content of organic matter is about 8 % in the technogenic layer of soddy-podzolic buried soil, possibly due to the presence of carbonaceous particles. The cation exchange capacity in runoff soils has average values and exceeds the absorption capacity of the background soil.
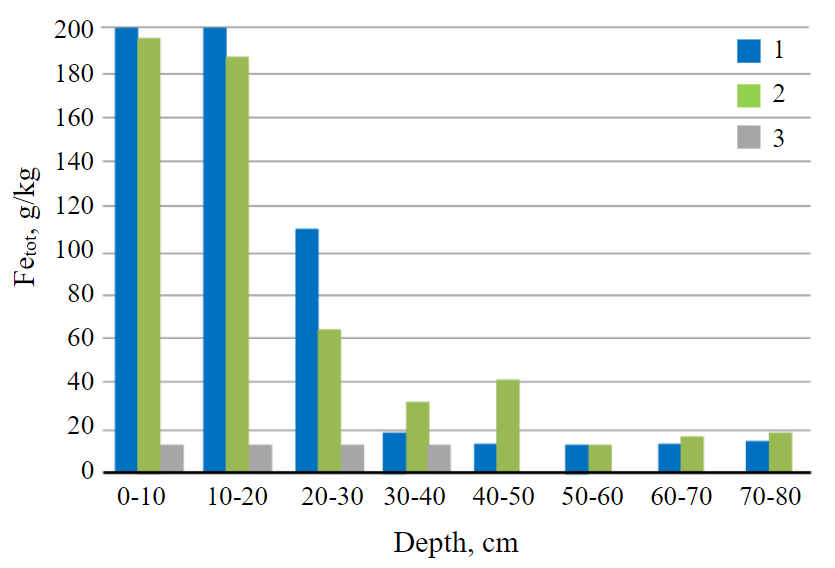
Fig.5. Total iron content 1 – soddy-podzolic soil buried under the technogenic layer; 2 – technosol; 3 – soddy-podzolic background soil
The exchangeable acidity and the content of Alex in the soils at the acid mine water discharge site are lower than in the background soils. This is due to the technogenic nature of acidity in the soddy-podzolic buried soil and technosol, namely, the presence of sulphide minerals. The technogenic layer of soddy-podzolic buried soil has a minimum value of exchangeable acidity along the profile from 0.75 to 0.81 mmol/100 g, while in the horizons of buried soddy-podzolic soil, exchangeable aci-dity corresponds to the background soil and is 6-8 mmol/00 g. The same trend is typical for Hex.
The introduction of sludge and waste from soda production into the technosol provides low values of exchangeable acidity from 0.09 to 2.00 mmol/100 g to a depth of 40 cm. The amount of exchangeable calcium in the 0-10 cm layer of the technosol exceeds this indicator in the background and buried soils. In the soddy-podzolic soil buried under the technogenic layer, the amount of exchangeable calcium increases with depth, while in the technosol, on the contrary, it decreases.
The content of total iron Fetot in the upper 20-cm soil layer in the non-reclaimed and reclaimed sites is 15 times higher (200 g/kg) than in the humus horizon of the background soil (Fig.5, Table 1). The iron content decreases to the background level with depth in the soddy-podzolic soil buried under the technogenic layer and amounts to 11-13 g/kg (Fig.5).
According to Table 1, Fetot does not migrate down the profile, but remains in the substance of technogenic deposits. The amount of Fetot in the background soil corresponds to its content in the soddy-podzolic buried soil from a depth of 30 cm, which also indicates the absence of migration of the unreclaimed site in the soil. In the technosol, its increased content was noted to a depth of 50 cm, which is associated with the introduction of sludge and waste from soda production to the upper layer during the reclamation; then the technogenic material of the dump and the introduced substances were mixed. This ensured the penetration of the technogenic substance into the middle layers of the technosol and the high content of Fetot relative to the background. The content of mobile iron in soils decreases with depth; there are no significant differences from the background.
The amount of Fetot in soils in the former discharge site of acid mine waters is explained by the mineralogical features of overburden rocks, the main part of the minerals in which are unstable and readily soluble iron sulphates [25].
Sulphate ions predominate in the water extract of soils, their content varies from 0.7 to 1.9 mmol/100 g, except for the surface layer of technosol, 7.4 mmol/100 g (Table 1). The content of mobile sulphur Smob in the studied soils does not differ and is hundreds of times higher than this indicator in the background soil. A correlation was found between the content of mobile sulphur and the content of total iron R2 = 0.71. The source of mobile sulphur in technogenic soils is technogenic deposits from rock dumps, the material in which contains sulphur in sulphide, sulphate, and elemental forms [32].
The study of the particle size distribution in the soddy-podzolic buried soil showed its clayey composition (Fig.6). In the 0-10 cm layer of soddy-podzolic buried soil, a significant proportion (30 %) was gravel, about 10 % of which has a size of 5-10 mm or more. This indicates the presence of deposits on the surface of the acid mine waters discharge site containing fragments of overburden. With depth, the amount of gravel decreases. The predominant fraction to 40 cm is particles with a size of 1-0.1 mm, below, a fraction with a size of 0.05-0.01 mm.
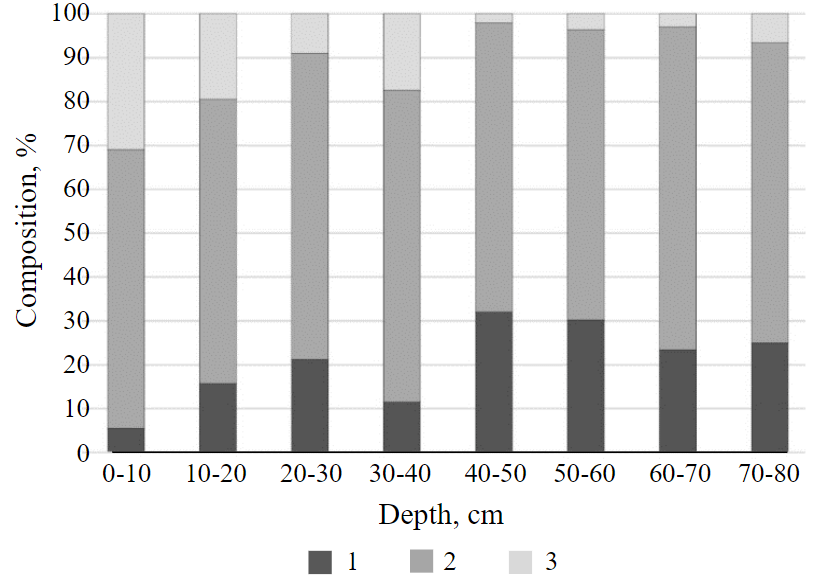
Fig.6. Particle size distribution of soddy-podzolic soil buried under the technogenic layer of soil 1 – physical clay; 2 – physical sand; 3 – gravel
The technosol also has a clayey composition, the presence of gravel in a 20-cm layer is noted, however, in a smaller amount than in the soddy-podzolic buried soil. Below 40 cm gravel is absent.
The properties of the buried soddy-podzolic soil correspond to those of the background soil. However, a 30-cm layer of technogenic deposits from the dump causes a sharply acid reaction of the soil environment pH H2O = 3.0, reduced values of exchange-able aluminium and calcium, and an increased content of total iron relative to buried and background soils. It can be assumed that the buried soddy-podzolic soil was in a preserved state, its environmental properties did not undergo significant transformation. During reclamation, the technogenic soil layer of the acid mine water discharge site was mixed with the addition of sludge and carbonate-containing waste, which caused the deposit substance to enter deeper soil layers, which caused an even greater variation in properties. In the technosol, the actual (pH H2O = 5.0) and hydrolytic acidity is lowered, sulphide minerals are neutralized in the upper part of the profile, the content of organic matter and exchangeable calcium is high, and the content of total iron is increased in most of the profile. Reclamation on the soils of the acid sulphate runoff site led to a decrease in acidity, an increase in the amount of organic carbon and absorption capacity. The improvement of soil properties in the reclaimed site led to the emergence of a stable phytocenosis.
Geochemical assessment of soils
The study of the content of trace elements (Cu, Zn, Co, Ni, Cd, Pb, V, Mn, Li, Be, B, Se, Al) included identifying the degree of contamination and distribution of trace elements along the profile of soils formed on the area of runoff from the coal dump, relative to background values, clarke according to Vinogradov, APC and the Igeo index.
The content of trace elements (Li, B, Fe, Co, As, Pb, Se) in the studied soils exceeds their background values (Table 2). This is due to the high content of trace elements in overburden rocks [33], as well as their high adsorption on iron hydroxides and organic matter. According to [34], heavy metals are sorbed on iron hydroxides; however, in organogenic soils, iron competes with heavy metals; iron hydroxides can be used as a geochemical sorption barrier for the accumulation of trace elements.
The content of Cu and Cd exceeds the approximate permissible concentrations (APC) for acid clayey soils; for V, an excess of MPC was found. For Zn, the excess is typical for a layer of 0-20 cm of the background soil, the excess for Co and Ni was found below the geochemical barriers in soils in the area of the runoff from the dump with depth along the profile.
The total contamination index Zc, calculated for all the studied trace elements for the surface layers (0-10 and 10-20 cm) of the soddy-podzolic soil buried under the technogenic layer and technosol, indicates the absence of contamination.
The Igeo index was calculated for each element in the root layers (0-10, 10-20 cm) using the background content in the soddy-podzolic soil (Table 3). The studied samples of the soddy-podzolic buried soil are slightly contaminated with Li, B, V, and Se. The remaining elements had values less than zero, which indicates the absence of contamination in the studied soils. Thus, Se forms the geochemical orientation of the soil cover of the gold ore deposit area, the main minerals in which are pyrite, chalcopyrite, iron hydroxides, etc. [36]. The sources of Li and V are coals, while the main carriers of Li are illite and montmorillonite [37]. Due to weathering at sulphide ore features, Se, Li, V, and B can enter natural features, for example, into soils, which explains their presence in the technogenic alluvial soil layer in the dump runoff area.
Table 2
Trace elements content in soils of runoff from the coal dump
|
Soil |
Depth, cm |
рН KCl |
Cu |
Zn |
Co |
Ni |
Cd |
Pb |
V |
Mn |
Li |
Be |
B |
Se |
Al |
Zc* |
|
Soddy-podzolic buried under the technogenic layer |
0-10 |
2.8 |
92.87 |
41.90 |
4.35 |
17.47 |
5.17 |
17.94 |
375.58 |
102.25 |
83.11 |
1.75 |
389.27 |
5.16 |
92.52 |
4.3 |
|
10-20 |
2.9 |
120.48 |
43.20 |
3.95 |
17.42 |
5.02 |
21.49 |
425.81 |
86.80 |
82.12 |
1.68 |
383.43 |
5.27 |
90.87 |
5.0 |
|
|
20-30 |
3.1 |
111.60 |
67.96 |
9.65 |
33.96 |
8.75 |
25.00 |
314.97 |
192.61 |
70.48 |
2.05 |
330.32 |
3.90 |
175.09 |
– |
|
|
30-40 |
3.1 |
102.72 |
92.71 |
15.35 |
50.49 |
12.49 |
28.52 |
204.14 |
298.42 |
58.83 |
2.41 |
277.22 |
2.54 |
259.31 |
– |
|
|
40-50 |
3.4 |
75.57 |
104.02 |
21.22 |
63.83 |
12.37 |
26.53 |
193.77 |
376.28 |
63.00 |
2.53 |
253.15 |
3.53 |
252.18 |
– |
|
|
50-60 |
3.6 |
66.60 |
92.84 |
21.44 |
60.76 |
11.01 |
25.00 |
174.95 |
455.23 |
58.53 |
2.98 |
242.54 |
1.50 |
243.43 |
– |
|
|
60-70 |
3.6 |
68.66 |
92.71 |
21.20 |
59.95 |
11.04 |
27.22 |
181.55 |
454.44 |
53.09 |
2.81 |
240.53 |
1.62 |
229.08 |
– |
|
|
70-80 |
3.6 |
64.76 |
89.28 |
24.96 |
57.35 |
11.61 |
31.13 |
190.58 |
7.82 |
47.66 |
2.86 |
215.34 |
1.99 |
221.62 |
– |
|
|
Technosol |
0-10 |
5.3 |
88.17 |
64.59 |
8.20 |
27.47 |
5.02 |
49.92 |
345.20 |
211.95 |
126.51 |
2.92 |
488.64 |
4.65 |
82.88 |
5.5 |
|
10-20 |
4.2 |
85.60 |
61.89 |
7.98 |
26.57 |
4.98 |
47.97 |
330.54 |
204.39 |
121.00 |
2.76 |
468.10 |
4.25 |
84.19 |
5.5 |
|
|
20-30 |
2.8 |
62.46 |
49.41 |
7.57 |
30.72 |
10.19 |
32.94 |
300.85 |
120.38 |
194.06 |
4.46 |
598.86 |
6.07 |
139.35 |
– |
|
|
30-40 |
2.8 |
71.17 |
53.09 |
9.13 |
37.40 |
10.75 |
30.00 |
303.38 |
112.03 |
249.31 |
5.87 |
711.17 |
4.47 |
146.68 |
– |
|
|
40-50 |
2.9 |
121.35 |
60.68 |
8.35 |
32.84 |
9.76 |
23.02 |
319.89 |
100.95 |
232.13 |
5.59 |
681.27 |
1.77 |
127.54 |
– |
|
|
50-60 |
3.1 |
79.61 |
83.93 |
18.44 |
60.68 |
10.49 |
25.40 |
204.55 |
641.08 |
74.77 |
2.64 |
333.30 |
1.33 |
285.55 |
– |
|
|
60-70 |
3.2 |
83.36 |
102.22 |
22.24 |
72.95 |
12.37 |
29.72 |
233.66 |
728.25 |
74.89 |
2.83 |
311.98 |
2.97 |
289.89 |
– |
|
|
70-80 |
3.5 |
72.30 |
106.10 |
25.40 |
75.56 |
9.49 |
28.09 |
242.79 |
573.03 |
71.69 |
3.23 |
283.38 |
1.96 |
262.14 |
– |
|
|
Soddy-podzolic (background) |
0-10 |
3.5 |
85.23 |
128.57 |
15.91 |
53.11 |
11.12 |
43.07 |
174.69 |
496.00 |
48.09 |
2.79 |
225.16 |
3.13 |
240.00 |
– |
|
10-20 |
3.5 |
79.25 |
126.99 |
15.88 |
53.13 |
11.21 |
33.53 |
176.53 |
511.51 |
44.66 |
2.54 |
221.45 |
3.40 |
226.94 |
– |
|
|
20-30 |
3.6 |
70.43 |
97.08 |
14.83 |
48.65 |
12.58 |
23.28 |
160.29 |
508.85 |
38.69 |
1.93 |
204.41 |
2.56 |
225.02 |
– |
|
|
30-40 |
3.7 |
68.08 |
99.73 |
20.29 |
65.23 |
10.74 |
21.95 |
175.05 |
399.31 |
41.80 |
1.95 |
184.34 |
1.79 |
226.71 |
– |
|
|
Clarke according to Vinogradov [35] |
– |
47 |
83 |
18 |
58 |
0.13 |
16 |
90 |
1000 |
– |
3.8 |
12 |
– |
80,500 |
– |
|
|
APC**/MPC |
– |
66/ |
110/ |
– |
40/ |
1/ |
65/ |
/150 |
/1500 |
– |
– |
– |
– |
– |
– |
|
Notes. Trace elements are highlighted in bold, for which an excess was found in KСB rivers and bottom sediments according to the report (On the state and environmental protection of the Perm Krai in 2020. Perm: Ministry of Natural Resources and Environment of the Perm Krai, 2021, p. 288); * – Zc calculated for layers 0-10, 10-20 cm (as root-inhabited) relative to the corresponding background values; ** – APC according to SanPin 1.2.3685-21 (pH KCl < 5.5).
Table 3
Results of statistical analysis of the geological accumulation index Igeo
|
Soil |
Li |
Be |
B |
Al |
V |
Mn |
Co |
Ni |
Cu |
Zn |
Se |
Cd |
Pb |
|
Technosol (medium) |
0.8 |
<0 |
0.5 |
<0 |
0.4 |
<0 |
<0 |
<0 |
<0 |
<0 |
<0 |
<0 |
<0 |
|
Soddy-podzolic, buried under the technogenic layer (medium) |
0.2 |
<0 |
0.2 |
<0 |
0.6 |
<0 |
<0 |
<0 |
<0 |
<0 |
0.1 |
<0 |
<0 |
|
Contamination level |
1 |
– |
1 |
– |
1 |
– |
– |
– |
– |
– |
1* |
– |
– |
* Only for soddy-podzolic buried soil.
It should be noted that there is no intensive technogenic migration of elements down the soil profile. Pollutants, mainly Li, B, Be, Fe, V, are concentrated in the surface layers of soils in the area of acid mine water discharge and actively migrate with planar runoff in the form of suspended particles, together with dump minerals, into accumulative landscapes, fall into rivers, concentrate in bottom se-diments, and migrate with water flows over long distances, as evidenced by the data in [4, 10, 38].
Conclusion
Due to the technogenic deposits, a soddy-podzolic soil buried under the technogenic layer was formed at the acid mine water discharge site, which, according to WRB, is defined as Epitechnoleptic Technosols (Supraalbic, Epithionic, Clayic, Phytotoxic, Skeletic). The profile and properties of a typical zonal soddy-podzolic soil are preserved under a 30-cm layer of technogenic deposits. The reclamation of this soil using activated sludge and alkaline waste from soda production led to the emergence of a technosol; according to WRB, the soil was diagnosed as Technosols (Clayic, Histic, Gleyic).
The soddy-podzolic soil buried under the technogenic layer has a sharply acid pH reaction H2O = 3, which is an indicator of the presence of sulphide minerals. The high content of organic matter is due to the presence of carbonaceous particles (8-4.7 % in the layer formed by technogenic deposits); the exchange acidity of the technogenic layer is low; average absorption capacity is 31-20 mmol/100 g. Technosol is characterized by a less acid pH reaction H2O = 4.5-5.5; the content of organic matter down to a depth of 50 cm varies from 19 to 65 %, which is due to reclamation activities. The cation exchange capacity is average, but the amount of exchangeable calcium in the technosol is higher than in the soddy-podzolic buried soil, as is the Smob content. The Fetot content in the upper 30-cm soil layer of the acid mine water discharge site varies from 66 to 200 g/kg, which exceeds the background values by 8-15 times.
A study of the microelement composition showed an excess of Li, B, Fe, Co, As, Pb, Se content in the studied soils over the background values. An excess of APC for Cu, Cd and MPC for V was also found. However, a comprehensive assessment of geochemical environmental state of the surface layers of soils in acid mine water discharge site based on the Zc and Igeo indices showed the absence of contamination by the studied trace elements, except for Li and B, as well as Se for the soddy-podzolic soil buried under the technogenic layer.
Reclamation led to an improvement in the physical and chemical properties of technogenic soils, a decrease in acidity and an increase in the organic matter content. Formation of a stable phytocenosis in the reclaimed site of acid mine water discharge indicates the effectiveness of this reclamation method.
References
- Ahirwal J., Maiti S.K. Assessment of soil properties of different land uses generated due to surface coal mining activities in tropical Sal (Shorearobusta) forest, India. CATENA. 2016. Vol. 140, p. 155-163. DOI: 10.1016/j.catena.2016.01.028
- Arefieva O., Nazarkina A.V., Gruschakova N.V. et al. Impact of mine waters on chemical composition of soil in the Partizansk Coal Basin, Russia. International Soil and Water Conservation Research. 2019. Vol. 7. Iss. 1, p. 57-63. DOI: 10.1016/j.iswcr.2019.01.001
- Xiaoyang Liu, Huading Shi, Zhongke Bai et al. Heavy metal concentrations of soils near the large opencast coal mine pits in China. Chemosphere. 2020. Vol. 244. N 125360. DOI: 10.1016/j.chemosphere.2019.125360
- Maksimovich N.G., Pyankov S.V. The Kizel coal basin: ecological problems and solutions. Perm: Perm State National Research University, 2018, p. 288 (in Russian).
- Chowdhury R.A., Sarkar D., Datta R. Remediation of Acid Mine Drainage-Impacted Water. Current Pollution Reports. 2015. Vol. 1. Iss. 3, p. 131-141. DOI: 10.1007/s40726-015-0011-3
- Singh Kh.N., Narzary D. Geochemical characterization of mine overburden strata for strategic overburden-spoil management in an opencast coal mine. Environmental Challenges. 2021. Vol. 3. N 100060. DOI: 10.1016/j.envc.2021.100060
- Welch C., Barbour S.L., Hendry M.J. The geochemistry and hydrology of coal waste rock dumps: A systematic global review. Science of the Total Environment. 2021. Vol. 795. N 148798. DOI: 10.1016/j.scitotenv.2021.148798
- Zhihong Tu, Qi Wu, Hongping He et al. Reduction of acid mine drainage by passivation of pyrite surfaces: A review. Science of the Total Environment. 2022. Vol. 832. N 155116. DOI: 10.1016/j.scitotenv.2022.155116
- Kostin A.S., Krechetov P.P., Chernitsova O.V., Terskaya E.V. Data on physico-chemical characteristics and elemental composition of gray forest soils (Greyzemic Phaeozems) in natural-technogenic landscapes of Moscow brown coal basin. Data in Brief. 2021. Vol. 35. N 106817. DOI: 10.1016/j.dib.2021.106817
- Ushakova E., Menshikova E., Blinov S. et al. Environmental Assessment Impact of Acid Mine Drainage from Kizel Coal Basin on the Kosva Bay of the Kama Reservoir (Perm Krai, Russia). Water. 2022. Vol. 14. Iss. 5. N 727. DOI: 10.3390/w14050727
- Shipilova A.M., Semina I.S. Features of physical properties of soil of technogenic landscapes of forest-steppe zone of Kuzbass. News of the Ural State Mining University. 2016. Vol. 3 (43), p. 25-28 (in Russian). DOI: 10.21440/2307-2091-2016-3-25-28
- Sahoo P.K., Equeenuddin S.M., Powell M.A. Trace Elements in Soils around Coal Mines: Current Scenario, Impact and Available Techniques for Management. Current Pollution Reports. 2016. Vol. 2. Iss. 1, p. 1-14. DOI: 10.1007/s40726-016-0025-5
- Grantcharova M.M., Fernández-Caliani J.C. Soil Acidification, Mineral Neoformation and Heavy Metal Contamination Driven by Weathering of Sulphide Wastes in a Ramsar Wetland. Applied Sciences. 2022. Vol. 12. Iss. 1. N 249. DOI: 10.3390/app12010249
- Hulisz P., Różański S.Ł., Boman A., Rauchfleisz M. Can acid sulfate soils from the southern Baltic zone be a source of potentially toxic elements (PTEs)? Science of the Total Environment. 2022. Vol. 825. N 154003. DOI: 10.1016/j.scitotenv.2022.154003
- Ye Yuan, Zhongqiu Zhao, Shuye Niu et al. Reclamation promotes the succession of the soil and vegetation in opencast coal mine: A case study from Robinia pseudoacacia reclaimed forests, Pingshuo mine, China. CATENA. 2018. Vol. 165, p. 72-79. DOI: 10.1016/j.catena.2018.01.025
- Yu Feng, Jinman Wang, Zhongke Bai, Lucy Reading. Effects of surface coal mining and land reclamation on soil properties: A review. Earth-Science Reviews. 2019. Vol. 191, p. 12-25. DOI: 10.1016/j.earscirev.2019.02.015
- Bragina P.S., Tsibart A.S., Zavadskaya M.P., Sharapova A.V. Soils on overburden dumps in the forest_steppe and mountain taiga zones of the Kuzbass. Eurasian Soil Science. 2014. Vol. 47. Iss. 7, p. 723-733. DOI: 10.1134/S1064229314050032
- Zástěrova P., Marschalko M., Niemiec D. et al. Analysis of Possibilities of Reclamation Waste Dumps after Coal Mining. Procedia Earth and Planetary Science. 2015. Vol. 15, p. 656-662. DOI: 10.1016/j.proeps.2015.08.077
- Fall L.A.C.A., Montoroi J.-P., Starh K. Coastal acid sulfate soils in the Saloum River basin, Senegal. Soil Research. 2014. Vol. 52. Iss. 7, p. 671-684. DOI: 10.1071/SR14033
- Hulisz P., Kwasowski W., Pracz J., Malinowski R. Coastal acid sulphate soils in Poland: a review. Soil Science Annual. 2017. Vol. 68. Iss. 1, p. 46-54. DOI: 10.1515/ssa-2017-0006
- Ushakova E., Menshikova E., Blinov S. et al. Distribution of Trace Elements, Rare Earth Elements and Ecotoxicity in Sediments of the Kosva Bay, Perm Region (Russia). Journal of Ecological Engineering. 2022. Vol. 23. Iss. 4, p. 1-16. DOI: 10.12911/22998993/146269
- Pyankov S.V., Maximovich N.G., Khayrulina E.A. et al. Monitoring Acid Mine Drainage’s Effects on Surface Water in the Kizel Coal Basin with Sentinel-2 Satellite Images. Mine Water and the Environment. 2021. Vol. 40. Iss. 3, p. 606-621. DOI: 10.1007/s10230-021-00761-7
- Karakulieva A.A., Kondrateva M.A. Properties of Embryoses of Coal Mines Dumps of the Kizelovsky Basin. Anthropogenic Transformation of Nature. 2018. N 14, p. 156-159 (in Russian).
- Berdinskikh S.Y., Botalov V.S., Romanov A.V., Zaitsev A.G. Agrochemical characteristics of the top layer of soil on coal heaps and the effect of clay formation on their natural overgrowth (on the example of the Kizelovsky coal basin). Environmental Safety in Conditions of Anthropogenic Transformation of the Environment, 21-22 April 2022, Perm, Russia. Perm State National Research University, 2022, p. 437-441 (in Russian).
- Menshikova E., Osovetsky B., Blinov S., Belkin P. Mineral Formation under the Influence of Mine Waters (The Kizel Coal Basin, Russia). Minerals. 2020. Vol. 10. Iss. 4. N 364. DOI: 10.3390/min10040364
- Menshikova E., Blinov S., Belkin P. et al. Dumps of the Kizel Coal Basin as a Potential Source of Rare and Rare-Earth Elements. Science and Global Challenges of the 21st Century – Science and Technology. Perm Forum 2021. Lecture Notes in Networks and Systems. Cham: Springer. 2022. Vol. 342, p. 352-361. DOI: 10.1007/978-3-030-89477-1_35
- Krasilnikova S.A., Blinov S.M. Consequences of discharge of acidic mine waters in the Kizel coal basin. Natural and technical sciences. 2017. N 11 (113), p. 153-154 (in Russian).
- Fernández-Caliani J.C., Giráldez M.I., Waken W.H. et al. Soil quality changes in an Iberian pyrite mine site 15 years after land reclamation. CATENA. 2021. Vol. 206. N 105538. DOI: 10.1016/j.catena.2021.105538
- Nekrasova A.E., Bobrenko E.G., Knyсh A.I., Sologaev V.I. Reclamation of waste dump open joint-stock Company “Mine “Capital” of the Kemerovo region. Bulletin of the Omsk State Agrarian University. 2016. N 1 (21), p. 154-160 (in Russian).
- Ghrefat H.A., Abu-Rukah Y., Rosen M.A. Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environmental Monitoring and Assessment. 2011. Vol. 178. Iss. 1-4, p. 95-109. DOI: 10.1007/s10661-010-1675-1
- Martinez L.L.G., Poleto C. Assessment of diffuse pollution associated with metals in urban sediments using the geoaccumulation index (Igeo). Journal of Soils and Sediments. 2014. Vol. 14. Iss. 7, p. 1251-1257. DOI: 10.1007/s11368-014-0871-y
- Maksimovich N.G., Meshcheryakova O.Yu., Berezina O.А. et al. Formation of acidic effluents from dumps of the Kizel coal basin (Perm Territory). Geological evolution of the interaction of water with rocks: Materials of the fourth All-Russian scientific conference with international participation. 17-20 August, 2020, Ulan-Ude, Russia. Ulan-Ude: Bulletin of the Buryat Scientific Center of the Siberian Branch of the Russian Academy of Sciences, 2020, p. 239-241. DOI: 10.31554/978-5-7925-0584-1-2020-239-241
- Candeias C., Ferreira da Silva E., Salgueiro A.R. et al. The use of multivariate statistical analysis of geochemical data for assessing the spatial distribution of soil contamination by potentially toxic elements in the Aljustrel mining area (Iberian Pyrite Belt, Portugal). Environmental Earth Sciences. 2011. Vol. 62. Iss. 7, p. 1461-1479. DOI: 10.1007/s12665-010-0631-2
- Yifan Lin, Jing Wang, Chunye Lin. Vertical changes in the geochemical distributions of iron, manganese and heavy metals in wetland soil cores from cold temperate zones in northeastern China. Journal of Hazardous Materials Advances. 2022. Vol. 6. N 100085. DOI: 10.1016/j.hazadv.2022.100085
- Kasimov N.S., Vlasov D.V. Clarkes of Chemical Elements as Comparison Standards in Ecogeochemistry. Bulletin of Moscow University. 2015. Series 5. Geography. N 2, p. 7-17.
- Mishankin A.Yu., Yazikov E.G., Filimonenko E.A., Sobyanin Yu.P. Mineral and geochemical features of the soil cover of the Vyun gold ore deposit (the republic of Sakha (Yakutia)). Bulletin of the Tomsk Polytechnic University. Geo Аssets Engineering. 2021. Vol. 332. N 11, р. 98-109 (in Russian). DOI: 10.18799/24131830/2021/11/3381
- Beilei Sun, Yunxia Liu, Lucie Tajcmanova et al. In-situ analysis of the lithium occurrence in the N 11 coal from the Antaibao mining district, Ningwu Coalfield, northern China. Ore Geology Reviews. 2022. Vol. 144. N 104825. DOI: 10.1016/j.oregeorev.2022.104825
- Fetisova N. Study of Migration Forms of Metals in Rivers Affected by Acid Mine Drainage of the Kizel Coal Basin. Bulletin of the Tomsk Polytechnic University. Geo Assets Engineering. 2021. Vol. 332. N 1, p. 141-152 (in Russian). DOI: 10.18799/24131830/2021/1/3007
