Development of composition and study of sorbent properties based on saponite
- 1 — Ph.D. Researcher Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 2 — Ph.D., Dr.Sci. Director of Scientific Center Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 3 — Research Laboratory Assistant Saint Petersburg Mining University ▪ Orcid ▪ ResearcherID
- 4 — Postgraduate Student Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
Abstract
The development of a comprehensive approach to preventing the pollution of natural objects is necessary due to the high requirements of environmental legislation for the discharge of industrial wastewater. Adsorbents are used in various industries to extract heavy metals from wastewater. In this study the possibility of using saponite clay as a raw material for the production of sorbent for the extraction of copper ions Cu2+ from industrial wastewater is considered, a recipe and technology of sorbent production are developed, and its chemical composition is established. It has been established that the optimum temperature for heat treatment of the sorbent and corresponds to 550 ºC, since at this temperature saponite extrudates acquire strength (strength 34.1 kg/mm2) and textural properties (specific surface area of pellets 22.803 m2/g), allowing them to be used as sorbents. The kinetics of molecular adsorption was studied using model solutions of copper (II) sulfate. The extraction efficiency of copper (II) ions from the model solutions is 93 %. Extraction efficiency of copper (II) ions from copper plating wastewater reaches 94 %. SEM results confirm the presence of metal on the sorbent surface.
Introduction
Enterprises that use the hydrometallurgical process of processing of nonferrous metal ores are characterized by high profitability of production of the target commercial products, so hydrometallurgy is quite widespread. However, such enterprises are a source of heavy metal pollution of wastewater, which, in most cases, is discharged directly into open water bodies [1]. Extraction of various metals and wastewater treatment is one of the most important tasks of the metallurgical industry, for which sorption methods using inorganic natural and synthetic mineral sorbents are widely used [2, 3]. The share of the use of sorbents of natural origin for industrial wastewater treatment is insignificant. Currently, research is being actively conducted on the development of sorption materials based on natural aluminosilicates: bentonite, montmorillonite, cambrian clay, etc. [4, 5].
There are 32.83 million tons of unclaimed reserves of saponite clay in Russia. The use of this raw material as a sorbent base is an understudied area, studies of saponite clay are mainly aimed at studying it as an ameliorant, building material [6-8].
Saponite is a clay mineral of montmorillonite group, a layered silicate with specific surface area Sss = 35-40 m2/g, pore volume Vpores = 0.40 cm3/g, the plasticity of the clay base is 28.4 %. Saponite has cation-exchange properties, so the development of a special sorbent composition and its application for extraction of Cu2+ cations and other heavy metals is an important task.
Statement of the problem
Analysis of the literature showed that the main characteristics of the sorbent are mechanical strength and chemical resistance, as well as sorption efficiency and low cost of obtaining a commercial product.
The sorbents for wastewater treatment have the following requirements: mechanical and chemical resistance; consistency of fractional composition; sorbent solubility in liquid, release of volatile components, separation of the absorbed substance on the alternative material, permeability; regene-ration ability with subsequent use of the recovered sorbent in the water treatment process.
In the interplanar distance of the saponite mineral, there are the cations Mg2+, Ca2+, which are responsible for the exchange complex, and water molecules [9]. It is known that the exchange capa-city of saponite is 0.68 mg-eq/g [10]. The presence of OH– groups in the mineral structure promotes the anion exchange [11]. The specific surface area of the sorbent is maximized due to acid activation of the clay mineral, and the introduction of peptisators contributes to the manifestation of lyophilic properties [12].
According to the analyzed data, the creation of an environmentally friendly method of producing clay sorbent for the purpose of its involvement in water treatment procedures after hydrometallurgical processes is an important task.
Materials and methods
Viscoplastic mass of decanted saponite clay of one of the deposits of the Arkhangelsk region was used as the basis of saponite. The main mineral component according to the X-ray phase analysis is represented by the following minerals, wt.%: dolomite (CaCO3·MgCO3) – 20, каолинит (Al4[Si4O10](OH)8) – 22, saponite (Ca0.25(Mg,Fe)3((Si,Al)4O10)(OH)2·nH2O) – 45, badellite ((Na,Ca)0.5 – 0.3Al2(Si,Al)4O10(OH)2·nH2O) – 14 [10, 13, 14].
Saponite clay sludge is decanted at the Flottweg plant, the pit cake moisture content before the acid activation procedure is 39 %. The process of acid activation with hydrochloric acid with a concentration of 0.1 N, exposure time of 1 h, alkaline neutralization with cement, peptization with ferric chloride (III) of the viscoplastic clay mass was carried out in a specialized stationary mixer [15]. Cylindrical sorbent granules with a diameter of 6 mm and a height of 5-10 mm were formed by extrusion. The obtained pellets were subjected to removal of free moisture at 21 °C for 24 h. Then the heat treatment of the pellets was carried out in a muffle furnace in the temperature range of 400-550 °C [16].
Evaluation of sorbent textural characteristics such as specific surface area, micro- and mesoporosity, total pore volume and pore size distribution was performed by capillary condensation on a NOVA 1000e Quantachrome gas sorption analyzer at 77 K in nitrogen medium both for the formed sorbent and for the saponite base [17]. To confirm the results obtained, the total pore volume in water was determined according to GOST 17219-71 “Active coals. Method for determining the total pore volume by water”. Radial crushing strength of extrudates was determined according to ASTM D6175.
As part of a study of the kinetics of ion-exchange adsorption, the calculation of the static volume capacity of the clay sorbent for the extractable ion by the titrimetric method in the presence of the indicator murexide with trilon B 0.05 M was carried out.
Determination of the adsorption activity of the obtained sorbent with respect to Cu2+ cations with calculation of the sorption isotherm was performed under static conditions [18]. In a 100 ml flask filled with the model solution, the sorbent was added in a ratio of 1/50 [19]. Copper sulfate solution with concentrations from 0.25 to 2.1 mg/dm3 was used as a model solution. The sorbent suspensions were stirred intensively for 20 min on a shaking apparatus, then left in a static state for 30 min with periodic shaking for 1 to 2 min. Time of the adsorption experiment was 50 min. Extraction efficiency of copper cations from solutions was determined according to GOST 4388-72 “Drinking water. Methods for Determination of Copper Mass Concentration” [20, 21].
Copper-containing wastewater after electroplating bath copper electrolyte based on potassium pyrophosphate (K4P2O7) was taken for sorbent testing.
The experiments were carried out as follows: a cylindrical and semi-cylindrical sorbent with a diameter of 6 mm was loaded into the laboratory filter columns with a filter plate (pores 100) with a volume of 300 ml. The volume of copper-containing wastewater with initial concentration of Cinitial = 18.0 mg/dm3 and pH = 8.64 in the filter column was 100 ml, adsorption time – 24 h, the mass of the sorbent in the filter column – 20 g.
The morphology of samples of sorbent granules before and after sorption was studied using an electron raster microscope of TESCAN Vega 3 [22].
Results and discussion
Chemical analysis of saponite clay and obtained sorbent is shown in Table 1. The results of the oxide composition showed that the most important major components of the material before and after treatment with chemicals followed by clay heat treatment are compounds of silicon, magnesium, calcium.
Table 1
Chemical composition of the sorbent base and molded sorbent after heat treatment
|
Sample |
Content, wt.% |
||||||||
|
SiO2 |
MgO |
CaO |
Fe2O3 |
SO3 |
K2O |
TiO2 |
Al2O3 |
Other |
|
|
Saponite clay before sorbent molding |
52.73 |
22.14 |
4.58 |
9.91 |
– |
1.68 |
1.52 |
5.73 |
1.71 |
|
Sorbent after heat treatment at 550 °C |
41.10 |
17.89 |
28.55 |
1.99 |
1.01 |
0.91 |
0.37 |
5.68 |
2.5 |
Textural and strength characteristics of the obtained sorbent depending on the thermal regime are presented in Table 2.
Table 2
Results of measuring the porosity of saponite base samples after high-temperature treatment
|
Sample |
Specific surface Sss, m2/g |
Total pore |
Pore diameter distribution, % |
Pellet strength, kg/mm2 |
||||
|
0-20 Å |
20-50 Å |
50-100 Å |
100-320 Å |
>320 Å |
||||
|
Saponite base |
30.352 |
0.21 |
0.081 |
12.57 |
10.67 |
11.9 |
64.76 |
14.1 |
|
Processing at Т = 400 °С |
36.653 |
0.61 |
0 |
0.51 |
0.43 |
0.51 |
98.4 |
23.6 |
|
Processing at Т = 500 °С |
21.192 |
0.48 |
0 |
0.44 |
0.92 |
0.5 |
98.54 |
22.3 |
|
Processing at Т = 550 °С |
22.803 |
0.44 |
0 |
3.86 |
5.0 |
5.68 |
85.45 |
34.1 |
|
Processing at Т = 600 °С |
18.497 |
0.49 |
0 |
3.06 |
4.9 |
4.49 |
87.55 |
44.96 |
From the data obtained it is clear that the specific surface area decreases [23] with increasing treatment temperature of the clay sorbent base, the diameter changes insignificantly in terms of pore distribution. Mechanical strength of the sorbent treated at 400-500 °C is lost when interacting with the model solution, the clay swells, this leads to clogging of the model solution with clay particles due to the fact that intercrystalline water from the clay particles does not fully come out. According to measurements of the mechanical crush strength of extrudates, we can conclude that the strongest sorbent granules are obtained at a firing temperature of 600 °C. However, treatment of the sorbent at 600 °C leads to sintering of aluminosilicate layers, which reduces the specific surface of the sorbent, in turn, the adsorption capacity of the sorbent decreases. Consequently, heat treatment to obtain the sorbent is usually carried out in the range of 500-550 °C.
Studies of the kinetics of molecular adsorption and static sorption capacity, limiting stage of the ion exchange process
The kinetic curve of copper ions sorption by the clay sorbent, as well as changes in their concentration depending on the contact time of solid and liquid phases, are shown in Fig.1. Initial concentrations of model solutions were not used in the construction of the kinetic dependence, they are presented in the legend of the histogram. The graph shows the dependence of the molecular adsorption kinetics from the titration, the starting point of the curves is the treated initial solution (0 min) with a different concentration due to the presence of impurities (used for the titration reaction).
Figure 1 shows that adsorption equilibrium is reached for the concentration of 0.0281 mol/l – at the 80th minute; for the concentration of 0.0155 mol/l at the 40th minute; for concentrations of 0.043 and 0.022 mol/l – at the 130th minute. Thus, by the 130th minute of the experiment the equilibrium is reached by all solutions with the considered concentrations of copper (II) ions at the ratio of the sorbent and the model solution 1:50 [24]. The value of sorption capacity reaches 1.2 mol/kg
The static exchange capacity (SEC) and rate constants (K) of the ion exchange reaction, corresponding to the slope tangent of the dependence for different concentrations [25, 26], are given in Table 3. The sorption of copper ions from the solution by the clay sorbent proceeds in accordance with the kinetic model of the first order, as demonstrated by the dependence lgC = f (t) for the concentration of copper ions 0.022 mol/l (Fig.2), with the coefficient of determination equal to 0.966.
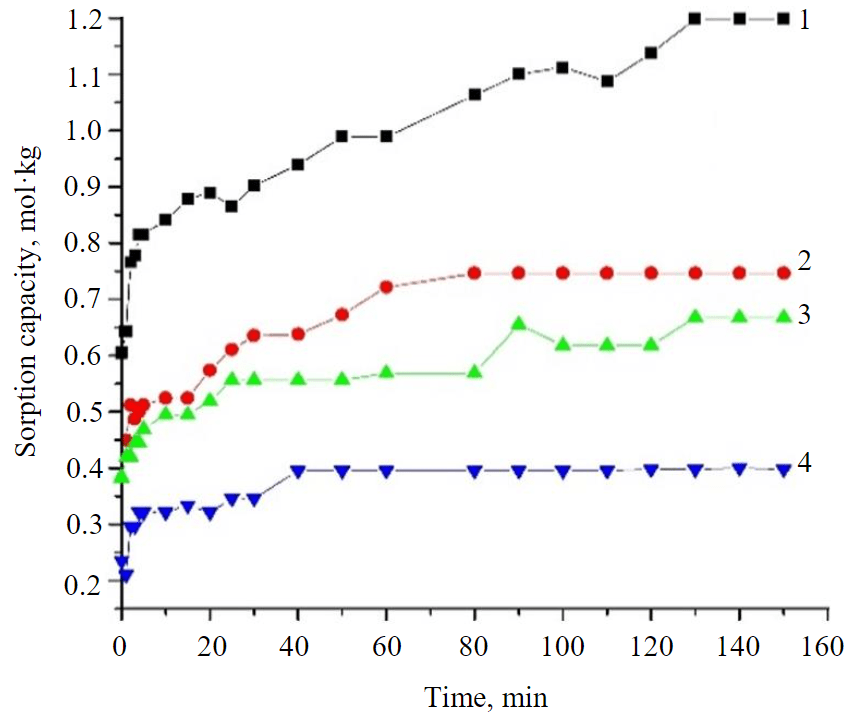
Fig.1. Kinetic curves of copper ions sorption on the clay sorbent at various initial concentrations of the model solution 1 – 0.043; 2 – 0.0281; 3 – 0.022; 4 – 0.0155 mol/l
Table 3
Calculated parameters of the model of Cu2+ adsorption by granules saponite sorbent in static conditions
|
C initial, mol/l |
Сfinal, mol/l |
α*, % |
SEC, mol/kg |
R2 |
K |
|
0.036 |
0.012 |
72.1 |
0.043 |
0.88 |
0.043 |
|
0.028 |
0.013 |
53.6 |
0.028 |
0.97 |
0.015 |
|
0.022 |
0.009 |
61.4 |
0.021 |
0.94 |
0.023 |
|
0.016 |
0.007 |
56.3 |
0.015 |
0.91 |
0.036 |
*α − current water treatment level.
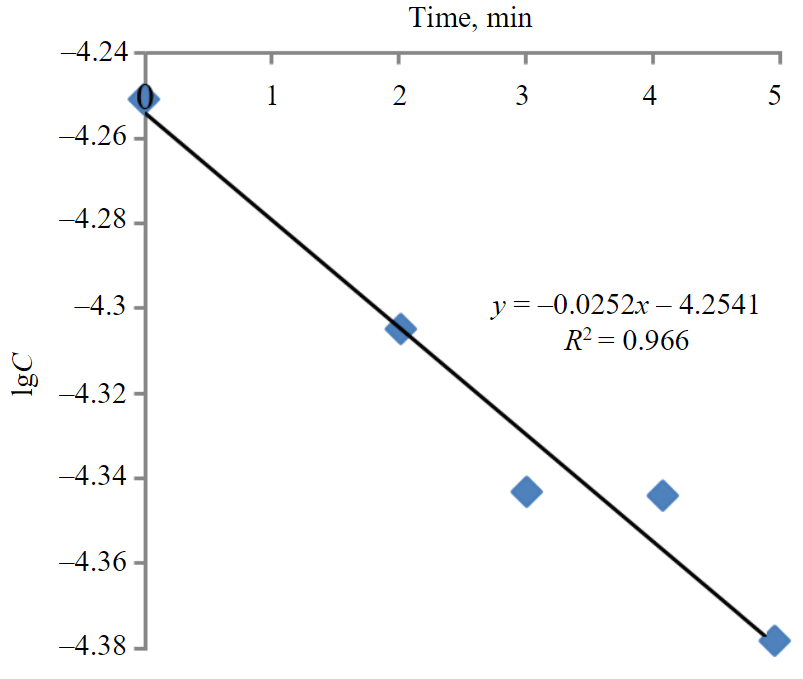
Fig.2. Dependence of lgC = f (t) for the solution with initial concentration 0.022 mol/l

Fig.3. The sorption process: initial soilution of galvanic waste (left), purified water with adsorbed copper (right)
The data obtained from the limiting stage of the process indicate a “film” kinetics and indicate external diffusion at high concentrations, which is visualized by the expiration of the sorption time on the surface of the pellets (Fig.3). The decrease in static volume capacity is consistent with the dependence of the reduction of SEC on the concentration of ions in the water [27].
The experimental data were analyzed using the kinetic model of first-order adsorption; it was proved that the rate of the chemical reaction does not depend on the concentration of Cu2+ ions and is determined by the ratio of the amount of sorbent and solution introduced (w:s); this is characteristic of the heterogeneous process taking place on the phase interface [28, 29].
According to the data obtained, the deposition of CuSO4 on the clay sorbent can be
described by the following scheme [30, 31]:
Therefore, multilayer “film” sorption is observed on the surface of the sorbent , firstly due to Cu(OH)2 – blue area on the sorbent, and then due to the low solubility of CuO – black area on the sorbent.
Determination of adsorption activity under static conditions
Studies of the sorption activity with respect to Cu2+ cation were carried out with the sorbent sample after thermal processing at 550 °C, the mechanical strength of the obtained sample is 34.1 kg/mm2, Sss = 22.8 m2/g.
A decrease in specific surface area due to the introduction of cement is observed when measuring the specific surface area of a molded laboratory sample thermally treated at 550 °C, but the strength characteristics of the sorbent pellet increase.
The degree of extraction of copper ions from the model solution with different concentrations under static conditions on the obtained sorbent and the purification efficiency are presented in Table 4.
It was found that the efficiency of sorption extraction of Cu2+ ions from the model solution was 86.3-100 % depending on the concentration. Parameters of the isotherm for the obtained sorbent sample are shown in Table 5, according to the obtained data the sorption isotherm is constructed (Fig.4) [32].
Table 4
Extent of extraction of copper (II) ions from the model solution
|
Cinitial, mg/dm3 |
Сfinal, mg/dm3 |
Adsorption efficiency, % |
|
0.25 |
0 |
100 |
|
0.5 |
0.05 |
99 |
|
1.25 |
0.07 |
94.6 |
|
1.75 |
0.17 |
86.3 |
|
2.1 |
0.23 |
89 |
According to data obtained on the Quantachrome NOVA analyzer, the isotherm is of type V-BET (Brunauer, Emmet and Teller), correlation coefficient R = 0.99, KBET = 7.176, is a multilayer adsorption isotherm, also related to type II and III isotherms, in which adsorption is limited to mesopores. In the case of the adsorption of a model solution on saponite clay, the adsorption mechanism is mono- and multilayer adsorption plus capillary condensation. It can be concluded that copper sorption proceeds first according to the Freundlich model and consists in filling the monomolecular layer, then the process proceeds according to the BET model and represents the formation of polymolecular layers.
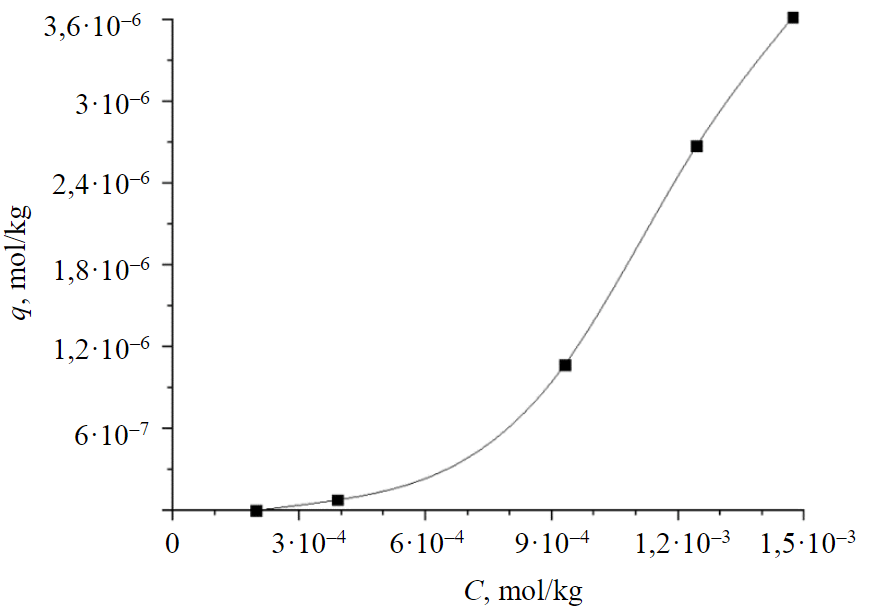
Fig.4. Isotherm of Cu2+ ion sorption on clayey saponite sorbent
Table 5
Experimental data on Cu2+ sorption on clay saponite sorbent
|
C0, mol/l |
C∞, mol/l |
q, mol/kg |
lgC |
lgq* |
1/C, mol/kg |
1/q, mol/kg |
ns·10–5, g/l |
Equation |
qmax, mmol/g |
|
3.94·10–6 |
1.57·10–10 |
1.97·10–4 |
–9.80 |
–3.70 |
6.35·109 |
5.08·103 |
1.25 |
q = 0.0143С0.1965 |
7.8·10–4 |
|
7.87·10–6 |
7.72·10–8 |
3.9·10–4 |
–7.11 |
–3.41 |
1.30·107 |
2.57·103 |
2.48 |
||
|
1.97·10–5 |
1.07·10–6 |
9.31·10–4 |
–5.97 |
–3.03 |
9.34·105 |
1.07·103 |
5.91 |
||
|
2.76·10–5 |
2.68·10–6 |
1.2·10–3 |
–5.57 |
–2.91 |
3.74·105 |
8.04·102 |
7.90 |
||
|
3.31·10–5 |
3.62·10–6 |
1.47·10–3 |
–5.44 |
–2.83 |
2.76·105 |
6.79·102 |
9.35 |
Notes: ns – amount of sorbed matter CuSO4 ; C∞ – current concentration of metal cation in solution, mol/l; С0 – initial concentration of metal cation in solution, mol/l; q – value of static exchange capacity, mol/kg; qmax – total static exchange capacity, mmol/g.
Approbation studies under dynamic conditions
Experiments on the adsorption of Cu2+ ions from wastewater after electroplating baths of copper plating from the production site were carried out, to determine the adsorption properties of the sorbent granules, close to industrial conditions [33, 34]. Prior to the experiment, the chemical composition of the source water for treatment was determined using an X-ray fluorescence analyzer. Six experimental series were conducted. The results before (initial concentration) and after (samples 1-5) the sorption treatment of wastewater are shown in Fig.5.
According to the results of measurements, we can conclude that the active adsorption of copper ions from the solution is carried out on the second-third day of the experiment, in addition to the sorption of copper-containing substance on the sorbent surface there is a partial sorption of phosphorus-containing compound, which is part of the electrolyte [35]. An increase in the content of iron compounds in water occurs after iron chloride, which is introduced into the sorbent as a peptizing additive, is washed out of the sorbent.
The results of spectrometric analysis of wastewater and treatment efficiency after sorption are presented in Table 6, the study for the accuracy of the results were conducted in parallel. The series number corresponds to the model solution passed through the columns for 6 days (with constant renewal of the solution). pH of the aqueous medium after wastewater passed through the sorbent layer was recorded simultaneously with the measurement of Cu2+ content in the treated effluent. The initial concentration is 18 mg/dm3.
The data obtained are consistent with Fig.3, adsorption on the surface of the sorbent is carried out in the first 2-3 days, then the process is less intense. Adsorption increases with increasing pH, the percentage of the sorbed ions increases sharply in a narrow pH range, complete saturation of the sorbent is achieved at pH above 9. Above this pH range and up to a pH of 11, copper solutions consist of positively charged ions (Cu2+, CuOH+), as well as neutral particles (Cu(OH)20, CuSO40), strongly acidic solutions consist of single cations Cu2+.
Table 6
Results of sorption purification studies
|
Series number |
Сfinal after sorption, mg/dm3 |
Adsorption efficiency, % |
рН |
|||
|
Column 1 |
Column 2 |
Column 1 |
Column 2 |
Column 1 |
Column 2 |
|
|
1 |
0.98 |
1.08 |
94.5 |
94 |
12.51 |
12.58 |
|
2 |
0.98 |
1.04 |
94.5 |
94.2 |
12.19 |
12.27 |
|
3 |
5.7 |
5.3 |
68.3 |
70.6 |
11.70 |
11.74 |
|
4 |
10.6 |
11.0 |
41.1 |
38.9 |
11.25 |
11.32 |
|
5 |
7.0 |
6.0 |
61.1 |
61.1 |
11.58 |
11.65 |
|
6 |
15.0 |
15.0 |
16.7 |
16.7 |
9.94 |
10.01 |
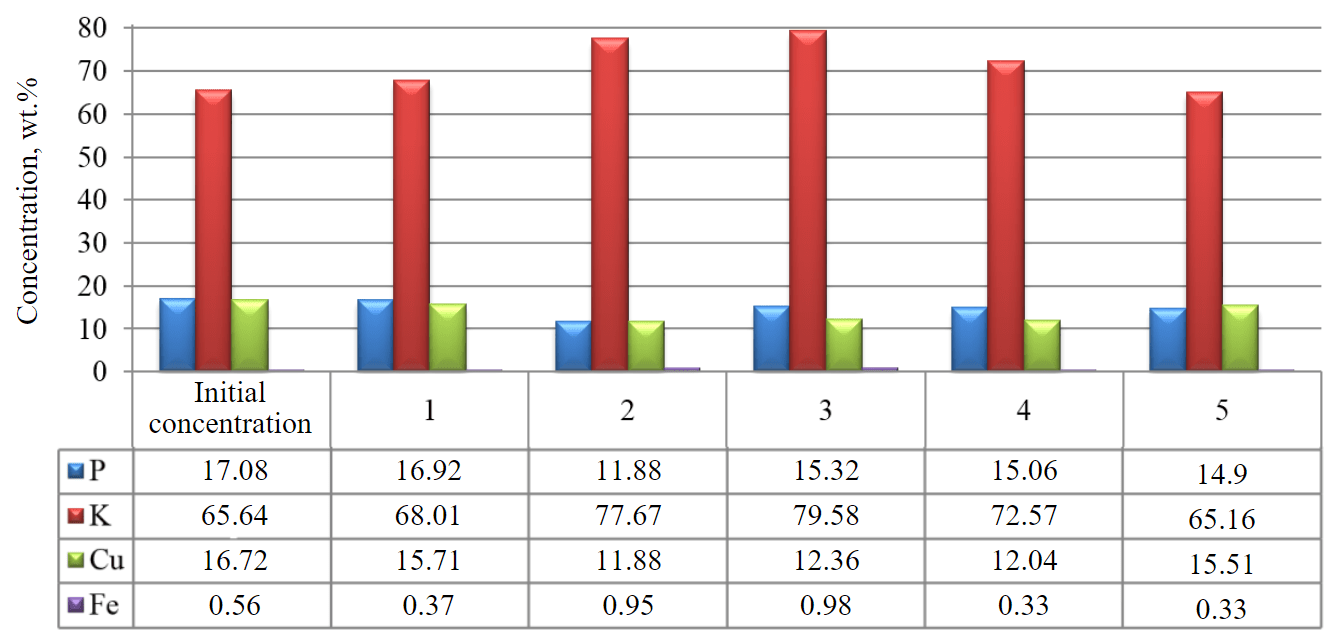
Fig.5. Histogram of remaining elements content in wastewater (wt.%) obtained on XRF (Epsilon) 1-5 – sample number
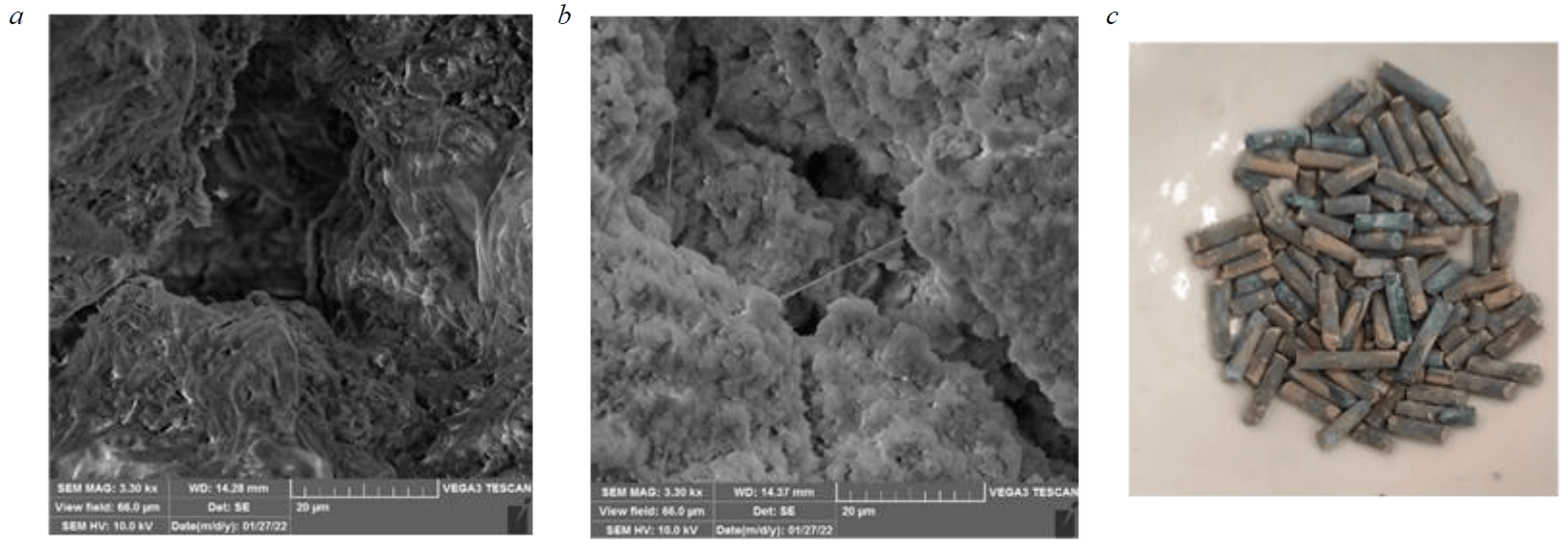
Fig.6. SEM images: saponite sorbent (a); saponite sorbent after sorption (b);
appearance of the sorbent after copper ions sorption (c)
Morphological studies of the sorbent
The microphotographs of the structures shown in Fig.6 demonstrate that pores form on the surface of the sorbent and, after adsorption, the saponite sorbent surface becomes rough and friable because of surface reactions. The SEM results of the solid product after adsorption agree with the results obtained in the previous study.
SEM images of the original saponite sorbent and the saponite sorbent that adsorbed copper are shown in Fig.6. Freshly formed sorbent has pores on the surface (Fig.6, a). Figure 6, b shows that the mass transfer of copper ions to the saponite sorbent surface occurs resulting in pore reduction due to filling of pores with adsorbed copper.
Conclusion
The article considers the prospects of application of clay sorbent based on saponite decanted sludge for the extraction of copper from waste water of industrial enterprises. The most significant research findings:
Decanted saponite clay is a viscoplastic material and the granules can be easily extruded into the optimal shape. Granules after heat treatment at 550 °C acquire satisfactory resistance (strength 34.1 kg/mm2) in interaction with the aqueous medium, as well as high relative porosity, the specific surface of the granules is 22.803 m2/g, which allows their use as an adsorbent for wastewater treatment.
The pellets have a sufficiently high adsorption capacity for copper ions (72 % under static conditions) and can be used to remove them from aqueous solutions at an ambient temperature of 21 °C.
The first order kinetic model satisfactorily describes the kinetics of copper adsorption. Metal adsorption by saponite sorbent granules is accurately described by type V isotherm. The adsorption mechanism is mono- and multilayer adsorption and capillary condensation.
The efficiency of Cu2+ removal from wastewater after the copper plating baths of the galvanic shop is clearly demonstrated by dynamic tests, the efficiency reaches 94 %.
The results of morphological studies indicate the presence of metals on the surface of the clay.
References
- Kagramanov G.G., Farnosova E.N., Lin M.M. et al. Removal of heavy metals from mine wastewater. Khimicheskaya promyshlennost segodnya. 2018. N 1, p. 44 (in Russian).
- Litvinenko V.S., Sergeev I.B. Innovations as a Factor in the Development of the Natural Resources Sector. Studies on Russian Economic Development. 2019. Vol. 30. Iss. 6, p. 637-645. DOI: 10.1134/s107570071906011
- Jaspal D., Malviya A. Composites for Wastewater Purification: A Review. Chemosphere. 2019. Vol. 246. N 125788. DOI: 10.1016/j.chemosphere.2019.12
- Koshelev А.V., Vedeneeva N.V., Zamatyrina V.A. et al. Development of Technology for Obtaining Sorbents based on Bentonite Clays for Water Purification Systems. Water and Ecology. 2018. N 2 (74), p. 32-39 (in Russian). DOI: 10.23968/2305-3488.2018.20.2.32-39
- Lebedev A.B., Utkov V.A., Khalifa A.A. Sintered Sorbent Utilization for H2S Removal from Industrial Flue Gas in the Process of Smelter Slag Granulation. Journal of Mining Institute. 2019. Vol. 237, p. 292-297. DOI: 10.31897/PMI.2019.3.292
- Pashkevich M.A., Alekseenko A.V. Reutilization Prospects of Diamond Clay Tailings at the Lomonosov Mine, Northwestern Russia. Minerals. 2020. Vol. 10. Iss. 6. N 517. DOI: 10.3390/min10060517
- Nakvasina E.N., Romanov E.M., Shabanova E.N. et al. The Use of Saponite-Containing Materials as Mineral Fertilizers at the Cultivation of Potatoes in Arkhangelsk Region. Bulletin of KSAU. 2019. N 1, p. 60-68 (in Russian).
- Oblitsov A.Y., Rogalev V.A. Prospective ways of diamondiferous rock enrichment wastes utilization at M.V.Lomonosov diamond deposit. Journal of Mining Institute. 2012. Vol. 195, p. 163-167 (in Russian).
- Besselink R., Stawski T.M., Freeman H.M. et al. Mechanism of saponite crystallization from a rapidly formed amorphous intermediate. Crystal Growth & Design. 2020. Vol. 20. Iss. 5, p. 3365-3373. DOI: 10.1021/acs.cgd.0c00151
- Minenko V.G. Justification and Design of Electrochemical Recovery of Saponite from Recycled Water. Journal of Mining Science. 2014. Vol. 50. N 3, p. 595-600. DOI: 10.1134/S106273911403020X
- Ivanov M.V., Chirkst D.E. Study of ionic exchange in soils for the purpose of their purification from heavy metals. Journal of Mining Institute. 2002. N 1 (150), p. 116-119 (in Russian).
- Eren E. Removal of copper ions by modified Unye clay, Turkey. Journal of Hazardous Materials. 2008. Vol. 159. Iss. 2-3, p. 235-244. DOI: 10.1016/j.jhazmat.2008.02.035
- Smyshlyaeva K.I., Rudko V.A., Kuzmin K.A., Povarov V.G. Asphaltene genesis influence on the low-sulfur residual marine fuel sedimentation stability. Fuel. 2022. Vol. 328. N 125291. DOI: 10.1016/j.fuel.2022.125291
- Kameshkov A.V., Kondrasheva N.K., Gabdulkhakov R.R., Rudko V.A. Comparison of coking additives obtained from different types of oil stock. Tsvetnye metally. 2020. N 10, p. 35-42. DOI: 10.17580/tsm.2020.10.05
- Atamanova O.V., Tikhomirova E.I., Kassymbekov Z.K., Podoksenov A.A. Improving the Sorption Ability of Modified Bentonite During Wastewater Treatment by Means of its Activation. Water and Ecology. 2020. N 1 (81). DOI: 10.23968/2305-3488.2020.25.1.3-12
- Petra L., Billik P., Melichová Z., Komadel P. Mechanochemically activated saponite as materials for Cu2+ and Ni2+ removal from aqueous solutions. Applied Clay Science. 2017. N 143, p. 22-28. DOI: 10.1016/j.clay.2017.03.012
- Litvinova Т., Kashurin R., Zhadovskiy I., Gerasev S. The Kinetic Aspects of the Dissolution of Slightly Soluble Lanthanoid Carbonates. Metals. 2021. Vol. 11. Iss. 11. N 1793. DOI: 10.3390/met11111793
- Xiaoliang Qi, Ruona Liu, Mengyu Chen et al. Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydrate Polymers. 2019. Vol. 209, p. 101-110. DOI: 10.1016/j.carbpol.2019.01.015
- Fedenko Yu.N., Pechonchik I.Yu. Influence of bentonite dose on the sorption extraction of Pb{2+} and Cd{2+}. Sovremennye tendentsii v razvitii vodosnabzheniya i vodootvedeniya: Materialy Mezhdunarodnoi konferentsii, posvyashchennoi 145-letiyu UP “Minskvodokanal”, 13-14 fevralya 2019, Minsk, Belarus'. Belorusskii gosudarstvennyi tekhnologicheskii universitet, 2019. Part 2, p. 118-119 (in Russian).
- Kurdiumov V.R., Timofeev K.L., Maltsev G.I., Lebed A. B. Sorption of nickel (II) and manganese (II) ions from aqueous solutions. Journal of Mining Institute. 2020. Vol. 242, p. 209-217. DOI: 10.31897/PMI.2020.2.209
- Cheremisina O.V., Cheremisina E.A., Ponomareva M.A., Fedorov А.Т. Sorption of rare earth coordination compounds. Journal of Mining Institute. 2020. Vol. 244, p. 474-481. DOI: 10.31897/PMI.2020.4.10
- Kudinova A.A., Poltoratckaya M.E., Gabdulkhakov R.R. et al. Parameters influence establishment of the petroleum coke genesis on the structure and properties of a highly porous carbon material obtained by activation of KOH. Journal of Porous Materials. 2022. Vol. 29, p. 1599-1616. DOI: 10.1007/s10934-022-01287-1
- Kanmani P., Rhim J.W. Nano and Nanocomposite Antimicrobial Materials for Food Packaging Applications, Progress in Nanomaterials for Food Packaging. Future Medicine. 2014, p. 34-48. DOI: 10.4155/ebo.13.303
- Cheremisina O., Litvinova T., Sergeev V. et al. Application of the Organic Waste-Based Sorbent for the Purification of Aqueous Solutions. Water. 2021. Vol. 13. Iss. 21. N 3101. DOI: 10.3390/w13213101
- Povarov V.G., Kopylova T.N., Sinyakova M.A., Rudko V.A. Quantitative Determination of Trace Heavy Metals and Selected Rock-Forming Elements in Porous Carbon Materials by the X-ray Fluorescence Method. ACS omega. 2021. Vol. 6. Iss. 38, p. 24595-24601. DOI: 10.1021/acsomega.1c00691
- Chirkst D.E., Litvinova T.E., Cheremisina O.V. et al. Isotherm of Strontium Sorption on Clay. Russian Journal of Applied Chemistry. 2003. Vol. 76, p. 727-730. DOI: 10.1023/A:1026053002075
- Bingjie Wang, Zhishan Bai, Haoran Jiang et al. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. Journal of Hazardous Materials. 2019. Vol. 364, p. 192-205. DOI: 10.1016/j.jhazmat.2018.10.024
- Alandis N.M., Mekhamer W., Aldayel O. et al. Adsorptive Applications of Montmorillonite Clay for the Removal of Ag(I) and Cu(II) from Aqueous Medium. Journal of Chemistry. 2019. N 7129014. DOI: 10.1155/2019/7129014
- Jianlong Wang, Xuan Guo. Adsorption kinetic models: Physical meanings, applications, and solving methods. Journal of Hazardous Materials. 2020. Vol. 390. N 122156. DOI: 10.1016/j.jhazmat.2020.122156
- Sen Gupta S., Bhattacharyya K.G. Adsorption of heavy metals on kaolinite and montmorillonite: a review. Physical Chemistry Chemical Physics. 2012. Vol. 14, p. 6698-6723. DOI: 10.1039/c2cp40093f
- Uddin M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chemical Engineering Journal. 2017. Vol. 308, p. 438-462. DOI: 10.1016/j.cej.2016.09.029
- Tangaraj V., Janot J.-M., Jaber M. et al. Adsorption and photophysical properties of fluorescent dyes over montmorillonite and saponite modified by surfactant. Chemosphere. 2017. Vol. 184, p. 1355-1361. DOI: 10.1016/j.chemosphere.2017.06.126
- Makisha N., Yunchina M. Methods and solutions for galvanic waste water treatment. International Science Conference SPbWOSCE-2016 “SMART City”, 15-17 November 2016, St. Petersburg, Russia. MATEC Web of Conferences, 2017. Vol. 106. N 07016. DOI: 10.1051/matecconf/20171060701
- Cardinale A.M., Carbone C., Consani S. et al. Layered Double Hydroxides for Remediation of Industrial Wastewater from a Galvanic Plant. Crystals. 2020. Vol. 10. Iss. 6. N 443. DOI: 10.3390/cryst10060443
- Ciesielczyk F., Bartczak P., Klapiszewski Ł. et al. Treatment of model and galvanic waste solutions of copper(II) ions using a lignin/inorganic oxide hybrid as an effective sorbent. Journal of Hazardous Materials. 2017. Vol. 328, p. 150-159. DOI: 10.1016/j.jhazmat.2017.01.009
