Dissolution kinetics of rare earth metal phosphates in carbonate solutions of alkali metals
- 1 — Ph.D., Dr.Sci. professor Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 2 — Postgraduate Student Saint Petersburg Mining University ▪ Orcid
Abstract
Treatment of apatite raw materials is associated with the formation of large-tonnage waste – phosphogypsum. The content of rare earth metals in such waste reaches 1 %, which makes it possible to consider it a technogenic source for obtaining rare earth metals and their compounds. Up to the present moment, there are neither processing plants, nor an efficient process flow to handle phosphogypsum dumps. It is rational to use a way that involves extraction of valuable components and overall reduction of phosphogypsum dumps. Such process flow is available with carbonate conversion of phosphogypsum to alkali metal or ammonium sulfate and calcium carbonate upon the condition of associated extraction of rare earth metal (REM) compounds. Associated extraction of REM compounds becomes possible since they form strong and stable complexes with hard bases according to Pearson, which among other things include carbonate, phosphate and sulfate anions. Formation of lanthanide complexes with inorganic oxygen-containing anions is facilitated by the formation of high-energy Ln-O bonds. The study focuses on the dissolution of lanthanide phosphates in carbonate media. It was established that formation of REM carbonate complexes from their phosphates is a spontaneous endothermic process and that formation of lanthanide carbonates and hydroxides serves as thermodynamic limitation of dissolution. A shift in equilibrium towards the formation of carbonate complexes is achieved by increasing the temperature to 90-100 °C and providing an excess of carbonate. The limiting stage of REM phosphate dissolution in carbonate media is external diffusion. This is indicated by increasing rate of the process with an intensification of stirring, first order of the reaction and the value of activation energy for phosphate dissolution from 27 to 60 kJ/mol. A combination of physical and chemical parameters of the process allowed to develop an engineering solution for associated REM extraction during carbonate conversion of phosphogypsum, which included a 4-5 h conversion of phosphogypsum at temperature of 90-110 °C by an alkali metal or ammonium carbonate solution with a concentration of 2-3 mol/l. As a result, a solution with alkali metal (ammonium) sulfate is obtained, which contains REMs in the form of carbonate complexes and calcium carbonate. The rate of REM extraction into the solution reaches no less than 93 %. Rare earth metals are separated from the mother liquor by precipitation or sorption on anion exchange resins, while the excess of alkali metal or ammonium carbonate is returned to the start of the process.
Introduction
Nowadays, development of many industries is directly related to the use of rare earth metals (REMs). The Russian Federation is among the top four countries in terms of the amount of explored REM re-serves; however, it cannot satisfy its own demand for individual REMs or a mixture of their com-pounds, which in turn leads to dependence on import producers (for example, China – the largest world producer of rare earths). A constantly growing demand for REM compounds requires handling of unconventional raw materials, for example, the products of processing the Kola Peninsula apatites.
The main part of geographically accessible REM reserves, located in the regions with a developed infrastructure, is found in the deposits, where they represent associated components [1, 8-11].
For example, the apatite-nepheline ores of the Khibiny group deposits in the Murmansk region contain over 40 % of Russian REM reserves, which under the current processing procedure remain in phosphogypsum dumps. In accordance with the state program of the Russian Federation “Development of the industrial sector and increasing its competitiveness to 2020”, development of a technology for REM extraction, separation and recovery, including the treatment of apatite and phosphogypsum, is a critically important strategic task.
Phosphogypsum is a by-product of processing phosphate ores (apatites, phosphorites) by the wet acid method, which currently accounts for more than 90 % of phosphoric acid production. According to various estimates, world production of phosphogypsum equals about 160-170 million tons per year. For each ton of phosphoric acid obtained from the phosphate ores, 4-5 tons of phosphogypsum are formed [15, 24, 26, 29].
Worldwide, more than 22 million tons of phosphoric acid are produced annually. The major amounts of phosphate ores are processed in the USA, former Soviet states, China, Africa and the Middle East, as well as in the East and South-East Europe. Phosphogypsum dumps play a key role in the extraction of REMs and other critical metals [25, 28].
The trend of European mineral policy for providing critical raw materials for sustainable non-polluting industries aims at involving phosphogypsum in the production process. Phosphogypsum contains about 0.5-1 % of REMs and other critical metals (Te, V, F, Ag, Mo, Se), which pass into phosphogypsum in the course of processing phosphate ores (for example, apatites) treatment [32-34].
The organization of phosphogypsum treatment can significantly reduce the criticality of these elements for many high-tech industries [40]. It is a well-known fact that many branches of the chemical industry face depletion of their raw material base, which increases the relevance of using processing waste and raw materials with a low content of valuable components in the production process. In addition, phosphogypsum dumps exert significant influence on the biological environment. For example, soils within a radius of up to two kilometers from phosphogypsum dumps become contaminated with waste compounds [17].
The mass fraction of REM oxides in phosphogypsum is lower than in bastnaesite ores or in the ores of the Bayan Obo deposit in China (this deposit is justly considered unique in terms of its REM content), but comparable to the mass fraction of REM oxides in less rich deposits. In terms of individual elements, the composition of rare earths is much better than in loparite and monazite due to a significantly higher content of scarce middle-group elements (europium, terbium, dysprosium) [2, 13].
Unlike other REM sources, phosphogypsum practically does not contain radioactive thorium. Thus, the treatment of apatite ores in the main production cycle and their long-term storage in tailing ponds can be considered a stage in the preparation of phosphogypsum for industrial processing.
The phosphate ores treatment can be carried out using both acidic and alkaline technologies, including treatment of monazite or xenotime concentrate with strong alkali or soda solutions [3].
All the options of acid treatment of phosphogypsum, aimed at extracting REM compounds, center around the stage of acid leaching using nitric [22, 30, 36], sulfuric [4, 5, 18, 31] or hydrochloric acids of various concentrations [14, 38]. The methods differ in the number of processing stages and the rate of REM extraction into the acidic solution, which does not exceed 80-85 %. However, neither of the options implies complete processing of phosphogypsum dumps, and some of the proposed engineering solutions can only worsen the ecological situation due to the use of organic extractants (for example, tertiary amines that belong to the 2nd hazard class) [21].
The reaction of carbonate conversion of gypsum to ammonium sulfate and calcium carbonate is well known since the beginning of the last century and serves as the basis for the majority of available engineering solutions for reducing the amount of phosphogypsum dumps using the technology of its carbonization [10].
Derivation of alkali metal (ammonium) carbonate by means of carbon dioxide absorption of the alkali or ammonium solution achieves the effect of binding one of the main greenhouse gases, which can be beneficially used for mitigating the consequences of global climate change and development of green technologies. Annual production of phosphogypsum (100-280 Mt) can bind 26-72 Mt of CO2, while already accumulated phosphogypsum dumps can handle noticeably larger amounts of gas [16, 23, 27].
Technologies of carbonate conversion of phosphogypsum in order to increase the processing depth can be augmented by the extraction of valuable components, for example, REMs and/or strontium salts [20, 37].
The disadvantages of the described methods include the multistage structure of the process, high costs of chalk roasting and subsequent dissolution-precipitation cycle. Nevertheless, the main advantage of the proposed methods for REM extraction during carbonate conversion of phosphogypsum is a decrease in the number of dumps and recovery of the valuable components.
Thus, development of an effective technology for processing phosphogypsum and recovery of REM concentrate and other marketable products is a relevant and promising task.
It is known that scandium can form soluble carbonate complexes composed of [Sc(CO3)2]– and [ScCO3]+ [6]. There is also a possibility of dissolving REM carbonates under the effect of excess ammonium or potassium carbonate [19, 25, 39]. However, the information on kinetic laws of dissolving poorly soluble REM salts in carbonate media is very sketchy.
The purpose of present research is to analyze the dissolution kinetics of REM phosphates in carbonate media.
Methodology
Thermodynamic analysis of REM phosphate solubility in carbonate media was carried out by means of mathematical modeling using the data on Gibbs energy of the formation of yttrium and lanthanide carbonate complexes at a temperature of 298 K, taken from literature sources [19, 25, 39].
To estimate thermal effects of carbonate complex formation at a temperature of 298 K, a well-known relation was used:
The entropy of complex ion formation for ordinary temperature conditions was calculated using the empirical Powell- Latimer equation [28]:
where М is the molar mass of the ion, g/mol; z is the charge of the ion; (r + x) is the radius of complex ion, calculated as a sum of thermochemical radii of the REM cation r and carbonate ion x, pm.
Information on thermochemical radii of yttrium and lanthanides was taken from paper [35]; thermochemical radius of the carbonate ion was assumed to be 155 pm [7].
Dissolution kinetics of REM phosphates in carbonate media was studied on model objects – REM phosphates obtained by means of inorganic synthesis [12] from nitrates of yttrium, cerium, neodymium, chemically pure erbium and ytterbium. Reliability of the obtained composition of REM compounds was confirmed by the analysis of precipitates using X-ray powder diffractometry and X-ray fluorescence spectroscopy. The choice of synthetic REM compounds was determined by phosphogypsum composition, which apart from cerium and yttrium contained REMs of the medium-heavy group, including neodymium. The behavior of yttrium REMs was studied on the example of yttrium and ytterbium phosphates.
The patterns of REM phosphate dissolution in carbonate media were studied in a temperature-controlled installation on the HEL Auto-MATE Reactor System platform with an adjustable stirring speed and reagent supply.
A weighted portion of the test material was placed in a glass vessel, a solution of potassium carbonate of specified concentration was added and stirred at a constant temperature, then the solid phase was separated by filtration on a vacuum filter at the temperature of the experiment. The aqueous solution was analyzed for the content of yttrium or lanthanides; the precipitate was dried to constant mass and weighed. Thus, the rate of extraction into the solution was controlled both by the gravimetric method according to a decrease in the mass of lanthanide phosphate and by lanthanide concentration in the aqueous solution according to the results of complexometric analysis of the solution in the presence of the arsenazo (III) indicator.
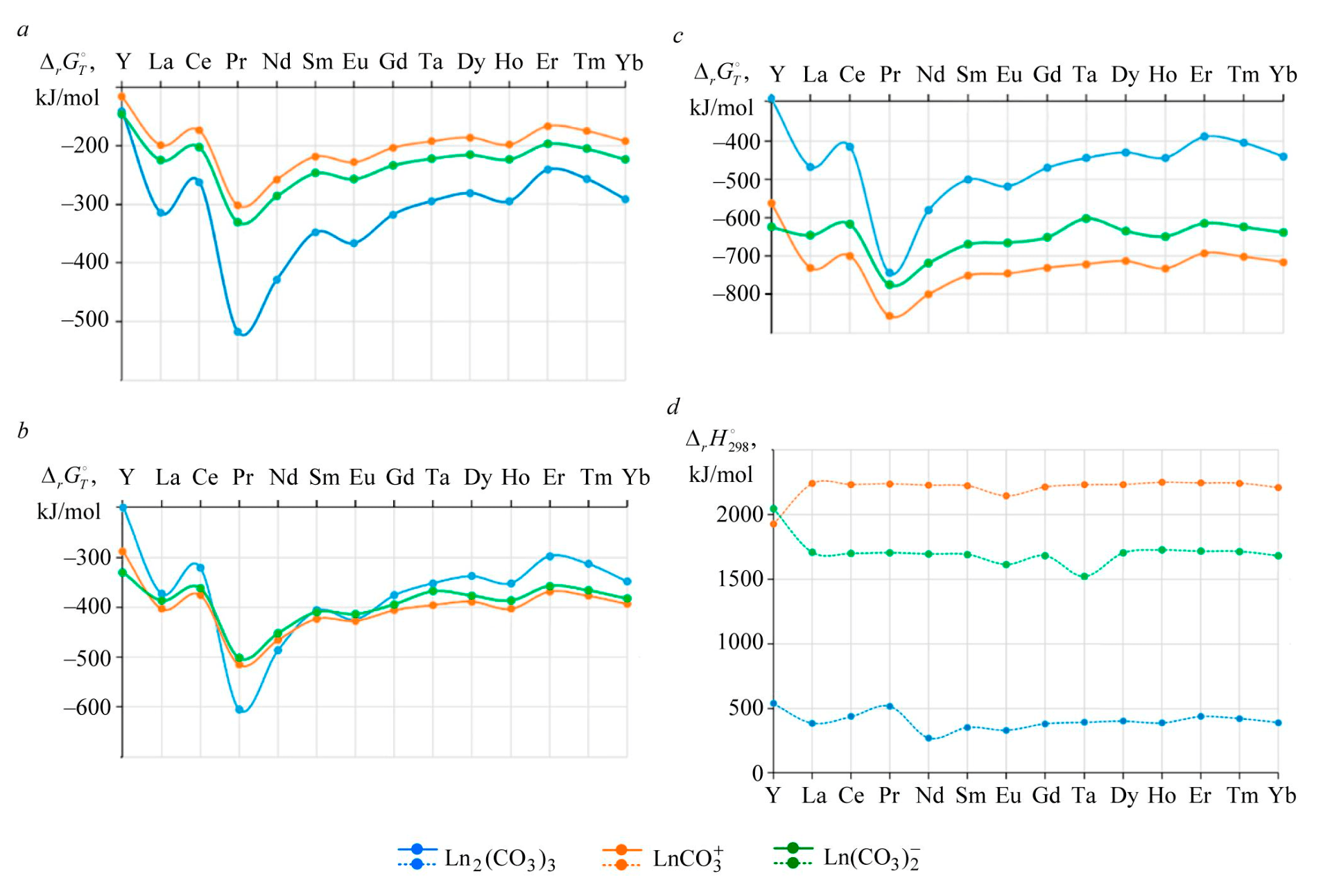
Fig.1. The effect of temperature on the formation of carbonate REM compounds: 25 °С (a), 50 °С (b), 90 °С (c), and enthalpy change (d)
The solution of potassium carbonate was prepared by dissolving a calculated amount of chemically pure compound in distilled water.
The rate of REM extraction into the solution was estimated by the formula:
where СLn – is the molar concentration of lanthanide ions in the final solution, mol/l; Vр is the volume of the solution, l; $M_\mathrm{LnPO_4}$ is the molar mass of lanthanide phosphate, g/mol; mi, mk are the initial and final masses of lanthanide phosphate quantity, respectively, g.
Calculation of thermodynamic parameters of lanthanide phosphate carbonization
Carbonization of lanthanide phosphates can occur in two ways:
- Formation of lanthanide carbonate from the phosphate:
and its subsequent dissolution with formation of a bicarbonate complex:
- Dissolution of lanthanide phosphate in the media of alkali metal carbonate with formation of mono- and bicarbonate complexes:
Figure 1 demonstrates the effect of temperature on the reaction behavior in both cases, calculated using the isobar equation of the chemical reaction.
The reactions of REM carbonate formation from the phosphate and dissolution of lanthanide phosphates with formation of carbonate complexes occur spontaneously and have an endothermic effect (Fig.1, d). The endothermic effect of carbonate complex formation is much greater than that of carbonate production.
Formation of yttrium and lanthanide hydroxides can become a thermodynamic hindrance to the dissolution of REM phosphates and formation of carbonate complexes:
Formation of carbonate complexes from lanthanide hydroxides in the following reactions:
occurs with much lower intensity compared to REM carbonates or phosphates, but due to the endo-thermic effect of the complex-forming reaction, as the temperature increases to 90-100 °С, the dis-solution process of REM hydroxides becomes spontaneous.
Under normal conditions, REM phosphate is more likely to be converted to carbonate or hy-droxide. As the temperature growing, increasing intensity of reactions accompanied by phosphate dissolution and formation of carbonate complexes.
Results
Due to heterogeneity of the process, dependence of the extraction rate on the stirring speed allows to assume the limiting stage for the reaction of lanthanide phosphate dissolution in carbonate media. The effect of stirring speed was obtained for solutions with a potassium carbonate concentration of 2 mol/l at temperature of 90 °C (Fig.2).
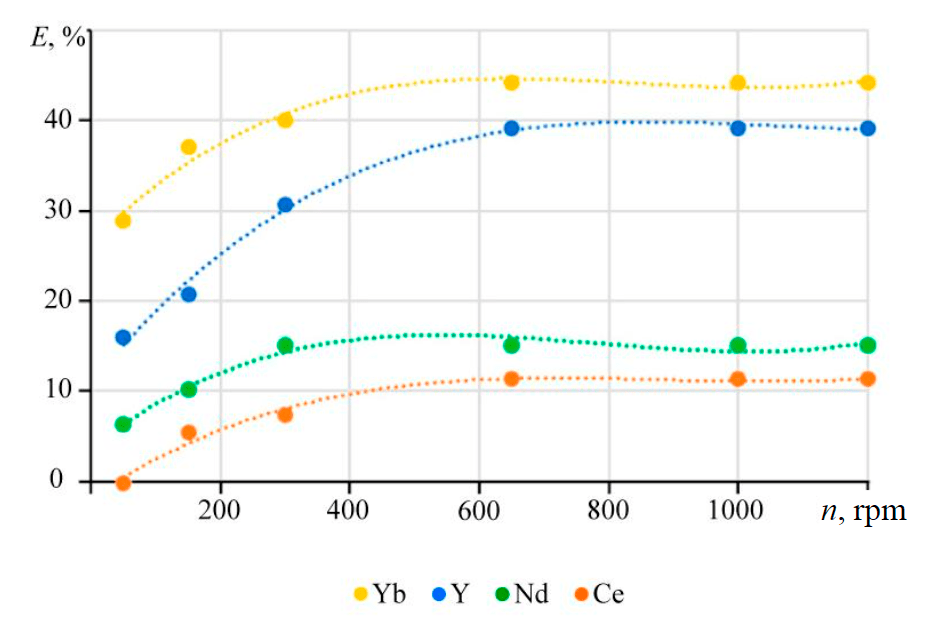
Fig.3. The effects of temperature and phase contact duration on the rate of REM extraction
he rate of extraction decreases from ytterbium to cerium, which among other things may be associated with differences in the activation energy for leaching the lanthanide phosphate. An increase in the stirring speed leads to an increase in the rate of extraction into the solution, which is typical for processes limited by external diffusion of the reagents.
Figure 3 demonstrates time dependences of the extraction rate for cerium (Ce, Nd) and yttrium (Y, Yb) REMs, obtained for temperatures of 50, 70 and 90 °С – i.e. the conditions more likely for REM carbonate formation and conditions, under which phosphate is more likely to dissolve and form carbonate complexes in the solution. Taking into account the experience of relatively few studies on the solubility of REM compounds in carbonate media, as well as the excess of alkali metal carbonate, recommended for carbonate conversion of phosphogypsum, the concentration of carbonate ion in the solution was 2 mol/l.
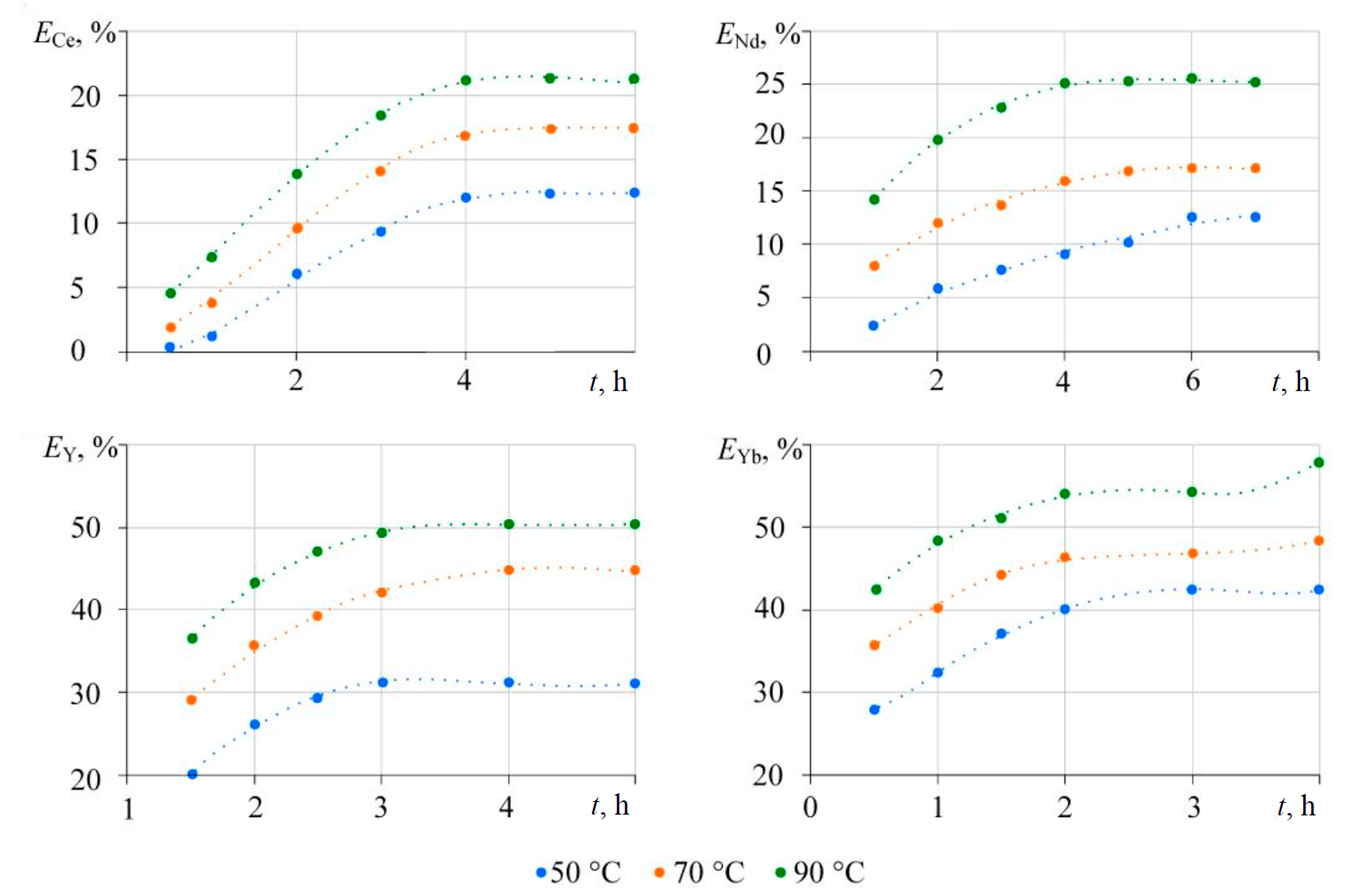
Fig.3. The effects of temperature and phase contact duration on the rate of REM extraction
Elements of the yttrium group have a higher rate of extraction into the solution than REMs of the cerium group. The equilibrium state is achieved at a phase contact duration of 2-3 hours for the yttrium-group REMs and 4-6 hours for the light lanthanides. Increasing temperature leads to in-creasing extraction into the solution, which can be attributed to thermodynamic specifics (endo-thermic effect of the process), as well as kinetic parameters of the reaction. For example, in case of neodymium, the time needed to reach a state close to equilibrium largely depends on the tempera-ture and decreases, as the temperature rises from 50 to 90 °C.
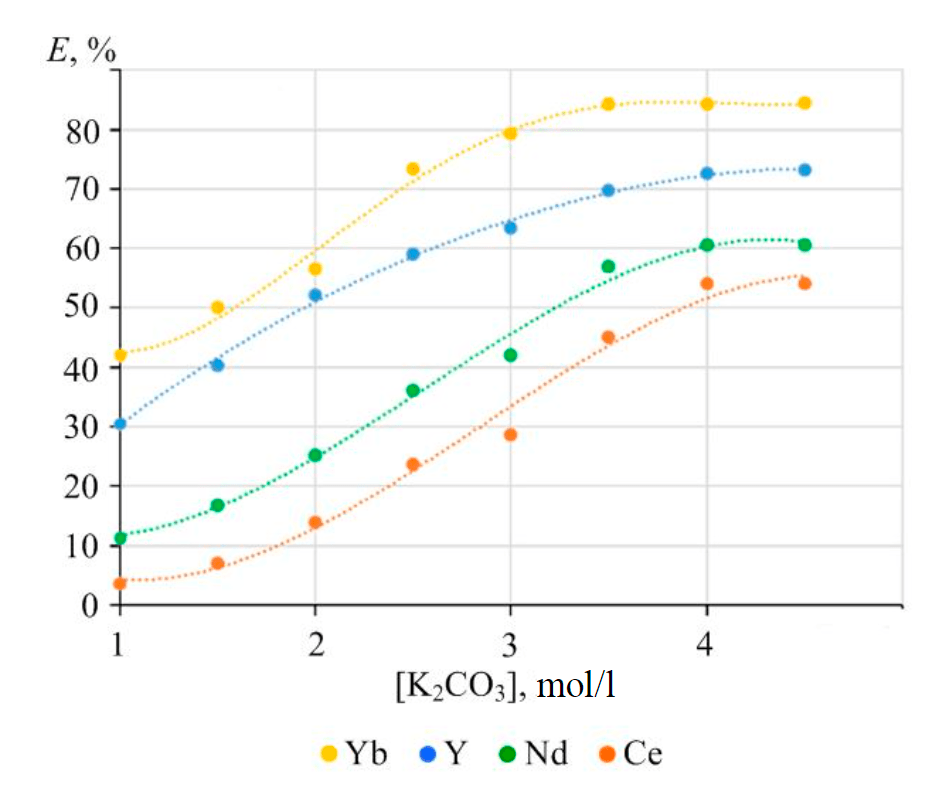
Fig.4. Dependence between the rate of lanthanide phosphate extraction into the solution and potassium carbonate concentration at a stirring time of 6 h and temperature of 90 °С
Figure 4 shows the results of the influence that the concentration of potassium carbonate solution exerts on the rate of REM extraction into the solution under conditions close to equilibrium, at a stirring time of 6 h. Further increase in the duration of the phase contact does not lead to any significant changes in solution concentration or in the mass of the residual lanthanide phosphate.
Increasing carbonate concentration in the solution leads to a consistent increase in the rate of REM extraction into the solution. The maximum extraction is obtained at a 4 mol/l concentration of potassium carbonate in the solution. Further increase in concentration does not result in any improvement. An increase in the extraction rate is observed during a transition from cerium to yttrium REMs.
Discussion
To describe the kinetics of the process, a model of stationary convective diffusion (model of effec-tive diffusion layer) was adopted. The reaction rate law takes the form of the first order equation:
Since there is an excess of potassium carbonate with respect to the REM phosphate, the rate of the process and the change of the sample mass are determined by the amount of lanthanide phosphate entering the complex-forming reaction:
where β – is the mass transfer coefficient; mt – is the mass of lanthanide phosphate.
Therefore, time dependences of the mass logarithm should be linear, with a slope corresponding to the coefficient of mass transfer. Semi-logarithmic time dependences of lanthanide phosphate mass, obtained as a result of studying the effect of temperature on the rate of lanthanide extraction into the solution, are shown in Fig.5.
Obtained dependences are adequately approximated by linear dependences presented in Table 1 with a determination coefficient no less than 90 %. As the temperature increases, so does the value of the slope (reaction rate constant).
Apparent activation energy of the process was derived from the temperature dependence of the conversion rate at an arbitrarily chosen (identical for all temperatures) degree of conversion in accordance with the equation:
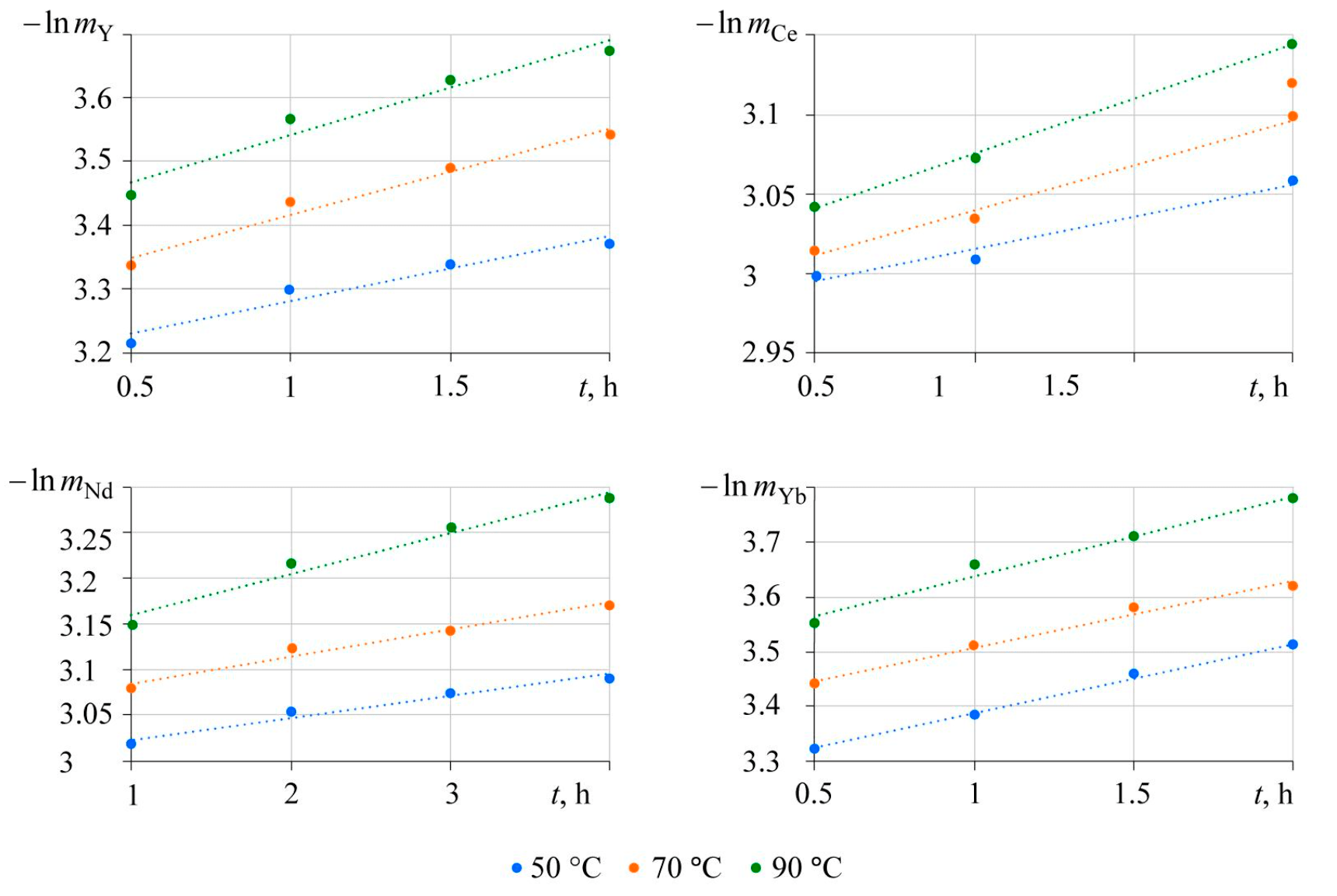
Fig.5. Time dependences of mass logarithm, obtained during the dissolution of lanthanide phosphate in a 2 M solution of potassium carbonate at different temperatures of the experiment
Table 1
Time dependence equations of the mass logarithm of lanthanide phosphate
|
Element |
Т, °С |
||
|
50 |
70 |
90 |
|
|
Yttrium |
–lnm = 0,1024t + 3,1782 |
–lnm = 0,1344t + 3,2828 |
–lnm = 0,1481t + 3,3936 |
|
Cerium |
–lnm = 0,0408t + 2,9745 |
–lnm = 0,0569t + 2,983 |
–lnm = 0,0691t + 3,0062 |
|
Neodymium |
–lnm = 0,0236t + 3,0003 |
–lnm = 0,0293t + 3,0553 |
–lnm = 0,0448t + 3,1145 |
|
Ytterbium |
–lnm = 0,1258t + 3,2624 |
–lnm = 0,122t + 3,3854 |
–lnm = 0,1452t + 3,4932 |
Taking into account the Arrhenius equation
или
Semi-logarithmic dependences of leaching rate on the inverse temperature are shown in Fig.6.
Linear dependences are approximated by the equations presented in Table 2 with a determination coefficient no less than 98 %.
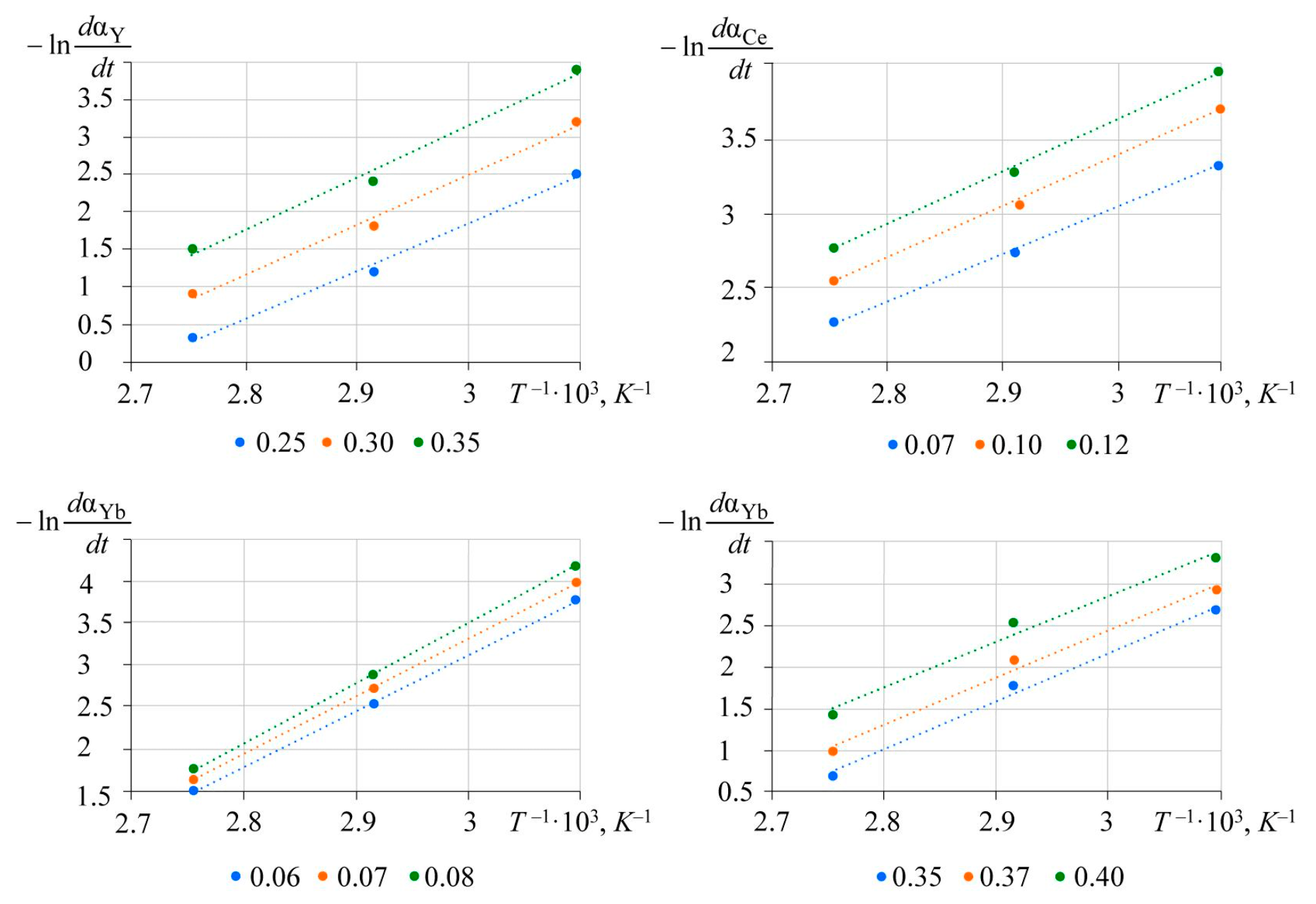
Fig.6. Logarithmic dependence of instantaneous reaction rate of REM phosphate dissolution
Table 2
Temperature dependences of reaction rate logarithm
|
α |
Yttrium |
α |
Neodymium |
|
0,25 |
ln(dα/dt) = –6397,8/T + 17,352 |
0,06 |
ln(dα/dt) = –6724,5/T + 17,045 |
|
0,30 |
ln(dα/dt) = –6727,9/T + 17,687 |
0,07 |
ln(dα/dt) = –6950,9/T + 17,536 |
|
0,35 |
ln(da/dt) = –7058/T + 18,022 |
0,08 |
ln(dα/dt) = –7177,4/T + 18,027 |
|
α |
Cerium |
α |
Ytterbium |
|
0,07 |
ln(dα/dt) = –3095,8/T + 6,2684 |
0,35 |
ln(dα/dt) = –5800,9/T + 15,227 |
|
0,10 |
ln(dα/dt) = –3353,1/T + 6,6829 |
0,37 |
ln(dα/dt) = –5671,7/T + 14,568 |
|
0,12 |
ln(dα/dt) = –3524,6/T + 6,9593 |
0,40 |
ln(dα/dt) = –5477,9/T + 13,579 |
The slope of the dependences is proportional to the activation energy. The values of apparent activation energy of lanthanides range from 30 to 61 kJ/mol, which is typical for diffusion or transient modes. Obtained values of activation energy and reaction orders are presented in Table 3.
Heterogeneous solid-liquid systems, where the dissolution of a poorly soluble compound takes place, are typically described by a first-order reaction, which has been confirmed experimentally. Presented kinetic data describe a complicated diffusion process of a complex-forming agent – a carbonate ion – to the surface of a lanthanide precipitate, dissociation and formation of a complex compound.
The difference in kinetic parameters of cerium from those of yttrium and other lanthanides can be explained by rapid oxidation to the tetravalent state, which leads to reduced yield into the solution in terms of thermodynamic parameters, despite lower activation energy compared to other elements.
Table 3
Kinetic parameters of lanthanide phosphate dissolution in carbonate media
|
Element |
Activation energy, kJ/mol |
k |
Arrhenius constant |
Apparent order of reaction n |
|
Y |
55,91 |
0,150 |
1,68⋅107 |
1 |
|
Ce |
27,63 |
0,070 |
6,68⋅102 |
|
|
Nd |
57,76 |
0,048 |
1,00⋅107 |
|
|
Yb |
46,95 |
0,155 |
8,91⋅105 |
Development of the engineering solution
Analysis of the combination of physical and chemical parameters of REM phosphate carbonization allowed to outline the technology of associated REM extraction during carbonate conversion of phosphogypsum. The processing of phosphogypsum by means of carbonate-alkaline method accompanied by associated REM extraction is a combination of the following operations (Fig.7): preparation of an alkali metal or ammonium carbonate solution; conversion of phosphogypsum; filtration, washing and drying of phosphochalk; REM separation from the carbonate-sulfate solution; separation of sulfate from alkali metal or ammonium carbonate.
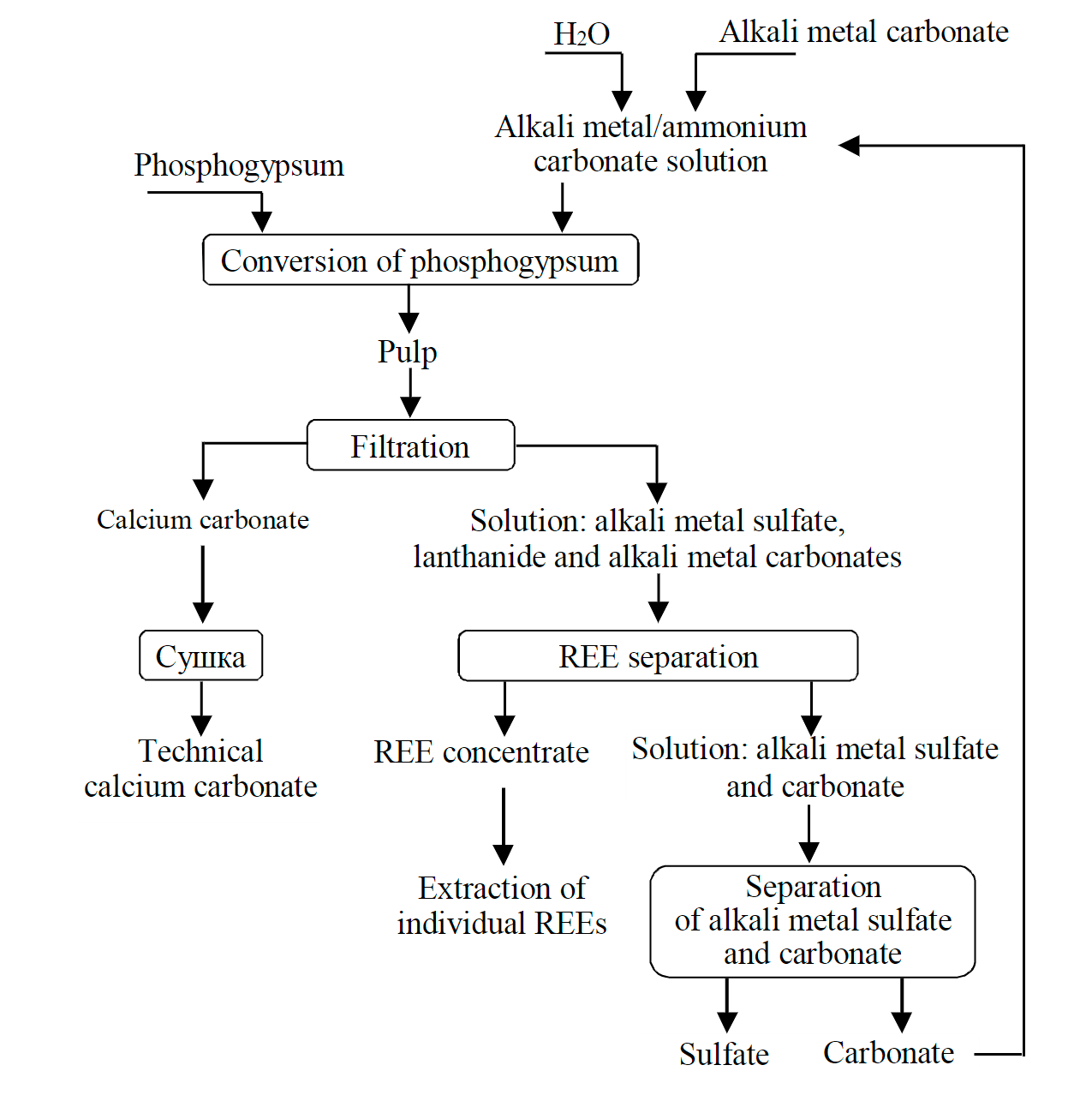
Fig.7. The scheme of complex phosphogypsum processing with associated REM extraction
Preparation of an alkali metal or ammonium carbonate solution can be performed in two ways: by dissolving industrial compounds or by absorbing carbon dioxide with ammonium or alkali hydroxide. In the latter case, a beneficial effect of CO2 utilization is achieved.
Phosphogypsum from the dumps is mixed for 4.5 ± 0.5 h with an alkali metal or ammonium carbonate solution with a concentration of 2.5 ± 0.5 mol/l at a temperature of 110 ± 10 °C and a liquid-to-solid ratio equal to 1900 ± 100 in respect to the sum of REM oxides in the phosphogypsum. Under given conditions, REM compounds pass into the solution in the form of carbonate complexes. The insoluble part of the pulp is represented by calcium carbonate, containing impurities of iron and other compounds of phosphogypsum. The conversion solution contains alkali metal (ammonium) sulfate, excess alkali metal (ammonium) carbonate and REMs in the form of carbonate complexes. The rate of REM extraction into the solution is 95 ± 3 %.
REMs can be separated from the carbonate-sulfate solution in the form of hydroxides by means of precipitation, as the solution is cooled to 20 ± 5 °C, or isolated by sorption on anion exchange resins or by liquid extraction using anion exchange extractants – for example, salts of quaternary am-monium bases.
After REM separation, the solution of potassium sulfates and carbonates is passed to the stage of separation between alkali metal (ammonium) carbonates and sulfates. During the conversion, potassium carbonate is used to extract potassium sulfate as a commercial product, whereas potassium carbonate in the form of a cycling solution is returned to the stage of solution preparation and conversion.
Conclusions
- A new approach to the processing of technogenic waste has been introduced; it is based on theoretical concepts of hard and soft acids and bases according to Pearson: REM cations, being hard acids, form strong anionic complexes with oxygen-containing inorganic ligands as hard bases, which among other things include carbonate ions.
- Thermodynamic patterns of carbonate conversion of poorly soluble REM compounds with the formation of carbonate complexes have been established. It has been demonstrated that dissolution of REM phosphates in carbonate media is a spontaneous endothermic process. Increasing temperature leads to intensification of REM phosphate dissolution with the formation of carbonate complexes.
- Formation of yttrium and lanthanide carbonates and hydroxides can serve as a thermodynamic limitation of REM phosphate dissolution in carbonate media. Dissolution of REM carbonates and hydroxides with the formation of carbonate complexes is an endothermic reaction, which prevails at a temperature of at least 90 °C in an excess of carbonate.
- The limiting stage of carbonate conversion of REM phosphates with the formation of carbonate complexes is external diffusion; the activation energy reaches 30-60 kJ/mol, but the possibility of intradiffusion processes is also conceivable.
- The sequence and conditions of technological operations have been determined for associated extraction of REM compounds during carbonate conversion of phosphogypsum to calcium carbonate and alkali metal (ammonium) sulfate.
References
- Kaplunov D.R., Radchenko D.N. Substantiation of the Full Cycle of Complex Subsoil Use in the Development of Solid Mineral Deposits. Gornyy informatsionno-analiticheskiy byulleten. 2011. N S1, p. 447-455 (in Russian).
- Kosynkin V.D., Selivanovskiy A.K., Fedulova T.T. et al. Comprehensive phosphogypsum treatment with the recovery of chemically precipitated chalk, gypsum, and ree concentrate. Tsvetnye Metally. 2012. N 3, p. 31-34 (in Russian).
- Mikhaylichenko A.I., Mikhlin E.B., Patrikeev Yu.V. Rare Earth Metals. Moscow: Metallurgiya, 1987, p. 232 (in Russian).
- Patent N 2225892 RF. Lokshin Eh.P., Vershkova Ju.A., Kalinnikov V.T., Tareeva O.A., lvlev K.G., Fedorov S.G., Pogrebnjak O.S. Method of recovering rare-earth minerals from phosphogypsum. Publ. 20.03.2004. Bull. N 8 (in Russian).
- Patent N 2293781 RF. Lokshin Eh.P., Kalinnikov V.T., Ivlev K.G., Levin B.V., Pogrebnjak O.S. Method of recovering rare-earth elements from phosphogypsum. Publ. 20.02.2007. Bull. N 5 (in Russian).
- Pyagai I.N., Kozhevnikov V.L., Pasechnik L.A., Skachkov V.M. Processing of alumina production red mud with recovery of scandium concentrate. Journal of Mining Institute. 2016. Vol. 218, p. 225-232 (in Russian).
- Ryabukhin A.G., Gruba O.N. Structural characteristics of carbonates of the double-charged cationsaem and 3D-elements (Mn-Zn). Bulletin of the South Ural State University. Series “Chemistry”. 2010. N 31, p. 83-89 (in Russian).
- Saveleva I.L. Rare Earth Industry of Russia: Current State, Resource Conditions of Development. Geografiya i prirodnye resursy. 2011. N 1, p. 122-129 (in Russian).
- Sarychev G.A., Strikhanov M.N. Rare earth metals raw materials sources development, program method and comprehensive approach to REM production facilities creation. Tsvetnye Metally. 2012. N 3, p. 45-52 (in Russian). DOI: 10.1134/S1875372811010112
- Sizyakov V.M., Nutrihina S.V., Levin B.V. Integrated technology phosphogypsum processing conversion method with ammonium sulfate, phosphomel and new products. Journal of Mining Institute. 2012. Vol. 119, p. 239-244 (in Russian).
- Ivanitskiy V.V., Klassen P.V., Novikov A.A. et al. Phosphogypsum and Its Use. Мoscow: Khimiya, 1990. p. 221 (in Russian).
- Cheremisina O.V. Kinetics of the processes of crystallization of compound of rare earth metals at inoculating phases. Tsvetnye Metally. 2009. N 10, p. 47-52 (in Russian).
- Al-Thyabat S., Zhang P. REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Journal of Mineral Processing and Extractive Metallurgy. Transactions of the Institutions of Mining and Metallurgy: Section C. 2015. Vol. 124. Iss. 3, p. 143-150. DOI: 10.1179/1743285515Y.0000000002
- Azimi G., Papangelakis V. Modelling of Calcium Sulphate Solubility in Concentrated Multi-Component Sulphate Solutions. Fluid Phase Equilibria. 2017. Vol. 260. Iss. 2, p. 300-315. DOI: 10.1016/j.fluid.2007.07.069
- Kulczyckaa J., Kowalskib Z., Smolc M., Herbert W.H. Evaluation of the recovery of Rare Earth Elements (REE) from phosphogypsum waste – case study of the WIZÓW Chemical Plant (Poland). Journal of Cleaner Production. 2016. Vol. 113, p. 345-354. DOI: 10.1016/j.jclepro.2015.11.039
- Hongtao Zhao, Huiquan Li, Weijun Bao et al. Experimental study of enhanced phosphogypsum carbonation with ammonia under increased CO2 pressure. Journal of CO2 Utilization. 2014. Vol. 11, p. 10-19. DOI: 10.1016/j.jcou.2014.11.004
- Szlauer B., Szwanenfeld M., Jakubiec H.W., Kolasa K. Hydrobiological characteristics of ponds collecting effluents from a phosphogypsum tip of the Police chemical works near Szczecin. Acta Hydrobiologica. 1990. Vol. 32, p. 27-34.
- Jarosinski A., Kowalczyk J., Mazanek C. Development of the Polish wasteless technology of apatite phosphogypsum utilization with recovery of rare-earths. Journal of Alloys and Compounds. 1993. Vol. 200. Iss. 1-2, p. 147-150. DOI: 10.1016/0925-8388(93)90485-6
- Johannesson K.H., Lyons W.B. Rare-earth element geochemistry of Colour Lake, an acidic freshwater lake on Axel Heiberg Island, Northwest Territories, Canada. Chemical Geology. 1995. Vol. 119. Iss. 1-4, p. 209-223. DOI: 10.1016/0009-2541(94)00099-T
- Kolokolnikov V.A., Kovalev M.I. Processing Rare-Earth Element Concentrate Obtained from Phosphogypsum. Chemistry for Sustainable Development. 2009. Vol. 17, p. 261-266.
- Kouraim M.N., Fawzy M.M., Helaly O.S. Leaching of Lanthanides from Phosphogypsum Waste using Nonyl Phenol Ethoxylate Associated with HNO3 and HCl. International Journal of Sciences: Basic and Applied Research. 2014. Vol. 16. № 2, p. 31-44.
- Lokshin E.P., Vershkova Y.A., Vershkov A.V., Tareeva O.A. Leaching of Lanthanides from phospho-hemihydrate with nitric acid. Russian Journal of Applied Chemistry. 2002. Vol. 75, p. 1753-1759. DOI: 10.1023/A:1022285330832
- Myung gyu Lee, Young Nam Jang, Kyung won Ryu et al. Mineral carbonation of flue gas desulfurization gypsum for CO2 sequestration. Energy. 2012. Vol. 47. Iss. 1, p. 370-377. DOI: 10.1016/j.energy.2012.09.009
- Mulopo J., Ikhu-Omoregbe D. Phosphogypsum Conversion to Calcium Carbonate and Utilization for Remediation of Acid Mine Drainage. Chemical Engineering and Process Technology. 2012. Vol. 3. Iss. 2, p. 1-6. DOI: 10.4172/2157-7048.1000129
- Ohta A., Kawabe I. Rare earth element partitioning between Fe oxyhydroxide precipitates and aqueous NaCl solutions doped with NaHCO3: Determinations of rare earth element complexation constants with carbonate ions. Geochemical Journal. 2000. Vol. 34. Iss. 6, p. 439-454. DOI: 10.2343/geochemj.34.439
- Parreira A.B., Kobayashi Jr A.R.K., Silvestre O.B. Influence of Portland cement type on unconfined compressive strength and linear expansion of cement-stabilized phosphogypsum. Journal of Environmental Engineering. 2003. Vol. 129, p. 956-960. DOI: 10.1061/(ASCE)0733-9372(2003)129:10(956)
- Pérez-Moreno S.M., Gázquez M.J., Bolívar J.P. CO2 sequestration by indirect carbonation of artificial gypsum generated in the manufacture of titanium dioxide pigments. Journal of Chemical Engineering. 2015. Vol. 262, p. 737-746. DOI: 10.1016/j.cej.2014.10.023
- Powell R.E., Latimer W.M. The Entropy of Aqueous Solutes. Journal of Chemical Physics. 1951. Vol. 19. N 1139. DOI: 10.1063/1.1748492
- Jiakuan Yang, Wanchao Liu, Lili Zhang, Bo Xiao. Preparation of load-bearing building materials from autoclaved phosphogypsum. Construction and Building Materials. 2009. Vol. 23. Iss. 2, p. 687-693. DOI: 10.1016/j.conbuildmat.2008.02.011
- Preston J.S., Du P. The recovery of a mixed rare-earth oxide and the preparation of cerium, europium and neodymium oxides from a South African phosphoric acid sludge by solvent extraction. Mineral Processing and Extractive Metallurgy Revue. 1988. Vol. 18. Iss. 2, p. 175-200. DOI: 10.1016/0304-386X(95)00067-Q
- Hammas-Nasri I., Horchani-Naifer K., Férida M., Barca D. Rare Earths Concentration from Phosphogypsum Waste by Two-Step Leaching Method. International Journal of Mineral Processing. 2016. Vol. 149, p. 78-83. DOI: 10.1016/j.minpro.2016.02.011
- Reijnders L. Cleaner phosphogypsum, coal combustion ashes and waste incineration ashes for application in building materials: A review. Building and Environment. 2007. Vol. 42. Iss. 2, p. 1036-1042. DOI: 10.1016/j.buildenv.2005.09.016
- Rutherford P.M., Dudas M.J., Samek R.A. Environmental impacts of phosphogypsum. The Science of the Total Environment. 1994. Vol. 149 Iss. 1-2, p. 1-38. DOI: 10.1016/0048-9697(94)90002-7
- Rutherford P.M., Dudas M.J., Arocena J.M. Heterogeneous distribution of radionuclides, barium and strontium in phosphogypsum by-product. Science of the Total Environment. 1996. Vol. 180. Iss. 3, p. 201-209. DOI: 10.1016/0048-9697(95)04939-8
- Shannon R.D., Prewitt C.T. Effective ionic radii and crystal chemistry. Journal of Inorganic and Nuclear Chemistry. 1970. Vol. 32. Iss. 5, p. 1427-1441. DOI: 10.1016/0022-1902(70)80629-7
- Preston J.S., Cole P.M., Craig W.M., Feather A.M. The recovery of rare earth oxides from a phosphoric acid by-product. Part1: Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy. 1996. Vol. 41. Iss. 1, p. 1-19. DOI: 10.1016/0304-386X(95)00051-H
- Vlasjan S.V., Voloshin N.D., Shestozub A.B. Producing calcium nitrate and rare-earth element concentrates by phosphogypsum conversion. Chemical Technology. Technology of inorganic materials. 2013. Vol. 64. № 2, p 58-62. DOI: 10.5755/j01.ct.64.2.6024
- Walawalkar M., Nichol C.K., Azimi G. Sustainable Processing of Phosphogypsum Waste Stream for the Recovery of Valuable Rare Earth Elements. REWAS. 2016, p. 107-112. DOI: 10.1007/978-3-319-48768-7_16
- Yu-Ran Luo, Byrne R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochimica et Cosmochimica Acta. 2004. Vol. 68. Iss. 4, p. 691-699. DOI: 10.1016/S0016-7037(03)00495-2
- Zhang P. 2014 Comprehensive Recovery and Sustainable Development of Phosphate Resources. Procedia Engineering. 2014. Vol. 83, p. 37-51. DOI: 10.1016/j.proeng.2014.09.010
