Analysis of the geochemical barriers effectiveness as the basis for the use of nature-like water purification technologies
- 1 — Ph.D., Dr.Sci. Professor Saint Petersburg State University ▪ Orcid
- 2 — Postgraduate Student Saint Petersburg State University ▪ Orcid
- 3 — Ph.D., Dr.Sci. Professor Saint Petersburg State University ▪ Orcid
- 4 — Ph.D. Associate Professor Saint Petersburg State University ▪ Orcid
- 5 — Bachelor Saint Petersburg State University ▪ Orcid
Abstract
Nature-like technologies are being introduced into many human activities including mining wastewater treatment. This work is based on long-term studies of the Sibay copper-zinc-pyrite deposit development. It is dedicated to assessment of geochemical barriers effectiveness in Cu, Zn, Cd removal from water of the Karagayly River (receiving quarry and dump drainage water). The research is based on the elements’ content and forms in water and bottom sediments, pH values etc. Four types of hydrogeochemical environment (formed due to changes in the water use over the past 20 years) were distinguished using discriminant analysis. The mechanisms of barriers formation and destruction were described. Statistical modeling of the metals’ precipitation was performed by multivariate regression analysis. Cu is adsorbed by recently formed Fe hydroxides, and, to a lesser extent, precipitates with sulfates as water pH increases. Antagonism to Mn hydroxides has been demonstrated, due to different physicochemical conditions for their precipitation. Zn enters solid phase mainly with sulfates, this element also forms its own mineral phases. The second mechanism is adsorption by recently formed Mn hydroxides, which corresponds to the idea of similar conditions for the precipitation of metal hydroxides. Cd behavior reflects conditions intermediate between these of Cu and Zn. Contribution of both mechanisms (related to Fe hydroxides and aqueous sulfates) is equal. Antagonism to Mn is absent. According to the assessment results using of nature-like technologies in situ in watercourses, canals and other water drainage systems is promising. Developed statistical models can be used for needs of experimental studies and artificial geochemical barriers engineering.
Funding
The research was supported by the Russian Science Foundation grant 22-77-00017
Introduction
Currently scientists pay great attention to looking for the most effective methods of removing metals from mining wastewater. Modern approaches are focused on complex methods preventing formation of secondary hazardous substances [1]. A promising direction is development of nature-like technologies. Actively discussed “gray-green”/“green-gray” technologies (CGGT) [2] as well as passive and alternative ones [3] are particularly suitable for cases of solid mineral resources extraction, which often don’t require use of chemicals for water purification. Therefore, nature-like approaches can be applied.
Non-ferrous metallurgy of the Southern Urals affects environment in a complex way [4]. Discharge of untreated wastewater and filtrate from dumps lead to small rivers degradation and the removal of heavy metals (Fe, Cu, Pb, Zn, Ni, Co, Cd, etc.) in the dissolved and suspended forms into higher-order rivers. Acidic mine, quarry and dump water decrease quality of water in rivers and lakes creating a potential threat to public health. Natural and artificial geochemical barriers reduce the migration ability of metals. Artificial barriers made of mining waste can also solve the problem of mineral resources rational using [5-7]. Geochemical barriers have several advantages such as feasibility of large-scale water purification projects in situ, no need for special equipment, relative inexpensiveness, implementing of several barrier types for different pollutants [8].
Artificial geochemical barriers are developed for wastewater treatment, they allow reducing of metal’s migration by means of sorption (sorption barrier) or precipitation caused by changes of pH (acidic and alkaline barriers) and Eh (oxidative and reductive barriers) values. Such barriers are often constructed using natural materials. Following materials are proposed for artificial barriers: natural mineral substances (carbonates [9, 10], layered and framework silicates and aluminosilicates [11-13], hydroxides [14], oxides [15], etc.) and their mixtures [16, 17], including those obtained from mining waste [7, 18, 19]. Peat [20] and ferromanganese nodules [21] can also act as natural sorbents. There are also wastewater treatment methods based on biogeochemical barriers and using indigenous aquatic and coastal aquatic plant species [22-24], and other biological agents, such as fungi and bacteria [25-27].
Many of the listed mineral components are present in bottom sediments under natural conditions and contribute to blocking migration of dissolved metals. Geochemical barriers may appear during technogenic impact, which leads to the deposition of pollutants in the impact zone. Considering the wide range of natural reagents that are used in water purification, combination of such reagents for every case requires careful justification. The main factors of metals’ phase transitions and deposition on barriers are the chemical composition of water, initial pH values, chemical properties of metals, their concentration and forms, hydrodynamic regime. These factors determine local hydrogeochemical conditions. Statistical modeling using mentioned data allows to recommend the most suitable reagents for water purification, taking into account local conditions. The modeling results can provide the basis for experimental and technological research required for in situ water purification systems engineering.
The purpose of the research is analysis of effectiveness of ore and related metals (Cu, Zn, Cd) removal from sub-dump filtrate and wastewater by the geochemical barriers in the Karagayly River (classified into several types); as well as statistical modeling of metal immobilization, which is the basis for development of nature-like technologies (not requiring specialized facilities) in mining industry.
Object and methods. Studied area is located near Sibay city (Republic of Bashkortostan) within the Krasnouralsk-Sibay-Gay copper ore zone. Devonian and Early Carboniferous volcanic and sedimentary rocks prevail [28]. Deposites of pyrite ore lies within contrasting volcanics of the Karamalytash complex (D2kr) [29], which is overlapped by the Ulutau (D2-3ul) and Kizil (C1-2kz) complexes. The latter contain Khudolaz limestone deposit (south-east from Sibay). The Sibay copper-zinc-pyrite deposit was developed from 1939 to 2020. In 1956 development of the Sibay quarry began. It required redirection of the Karagayly River flow into the Kamyshly-Uzyak River (in 1957). The old river bed downstream from the quarry retained the former name and was used to drain dump and mining water (Fig.1).
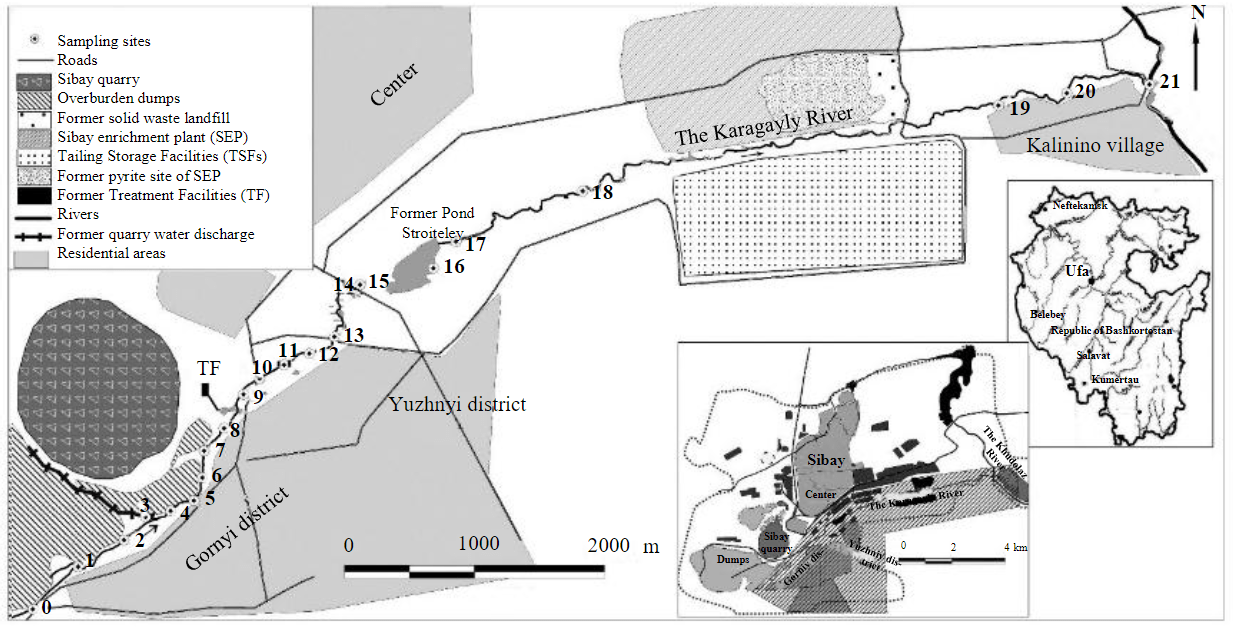
Fig.1. Scheme of water and bottom sediment sampling sites on the Karagayly River (according to [30] with additions)
During the development of the Novo-Sibay deposit sub-dump drainage and mining water formed the Karagayly River flow. Sub-dump sulfate-magnesium water (mineralization 2.5-9.0 g/l) is a pro-duct of mixing of infiltrated rainwater and groundwater discharged in the same place. In summer the average flow here over the past eight years is about 25 l/s (from 7 to 48 l/s depending on the rainfall). The mining water (quarry water) was discharged 1000 m downstream. The average wastewater flow reached 50-70 l/s. The Karagayly River flows east along the southern outskirts of Sibay and around 10 km downstream flows into the Khudolaz River. In the lower reaches of the river several tailing storage facilities (TSF’s) are located (Fig.1). Two new sections, including the one in use, are lined with geotextiles, which prevents water infiltration and its entry into the Karagayly River. Despite the lack of modern isolation materials in old sections of TSF’s, no water infiltration and no effect on pH and composition of river water were found. Small amount of acidic drainage water leaves tailing dam (pH of 4.1-4.4), but it does not reach the Karagayly due to relief peculiarities. pH values of the pulp discharged into the tailing dumps is 11.2-11.5. Described water regime existed for more than half a century. However, in the last 15 years, changes of water use led to the transformation of the existing geochemical barriers and pollutants flows.
In 2011, a quarry water treatment facilities were launched below the dicharge. Purified alkaline water was discharged into the river for several years. In 2015-2016 dredging work was carried out in mid- and lower reaches (from the treatment facilities downstream to the Kalinino village near the Karagayly mouth). They disturbed a layer of anoxic sediments accumulated since 1956. In winter of 2018-2019, a pyrite deposit ignited in the Sibay quarry. Under unfavorable weather conditions air was polluted with sulfur dioxide. The quarry was flooded to stop the fire. Therefore, in 2019, the discharge of quarry water into the Karagayly River was ceased and treatment facilities were stopped.
Research of the Karagayly River including sampling of water and bottom sediments has been carried out since 2004, on the basis of sampling sites network (Fig.1). Metal content in water and sediments initially was measured by atomic absorption spectroscopy (AAS) at VNIIOkeangeologia. Since 2014, samples of bottom sediments and water have been analyzed by mass spectrometry with inductively coupled plasma (ICP-MS) at Karpinsky Institute (VSEGEI). In both laboratories, complete acid decomposition of bottom sediment samples was carried out. Content of many metals and metalloids was measured, but in this research we focus on Cu, Zn, Cd, Fe, Mn, Co, Ni, Pb and Cr, monitored during the entire study period.
Mobile forms of metals were extracted with an ammonium acetate buffer solution (pH 4.8). Elements’ forms were studied using sequential extraction [30]. Six fractions were identified (residual does not count): exchangeable, carbonate and easily degradable organic matter, oxidizable, reducible and fraction of crystalline Fe hydroxides. The extracts were analyzed at the Resource Center of Saint Petersburg State University “Chemical Analysis and Materials Research Center” (analyst V.N.Grigoryan).
In the geoecological monitoring laboratory of Saint Petersburg State University, organic carbon (Corg) content in bottom sediments was measured using I.V.Tyurin’s method. Granulometric analysis of sediments was carried out using sieve and pipette methods. Biotesting of bottom sediments was accomplished using Daphnia magna Straus. (PND F T 14.1:2:3:4.120-06 T 16.1:2:2.3:3.9-06) and Chlorella vulgaris Beijer. (PND F T 14.1:2:3:4.10-04 T 16.1:2:2.3:3.7-04). Exposure time (acute toxicity) for Daphnia magna was 48 h, for Chlorella vulgaris – 22 h.
Scanning electron microscopy was carried out in the Resource Center for Microscopy and Microanalysis of the Scientific Park of Saint Petersburg State University (desktop scanning electron microscope TM 3000 (Hitachi, Japan) with energy dispersive microanalysis attachment OXFORD) in reflected electron mode.
Statistical processing of geochemical data (descriptive statistics, discriminant and variance analyses, as well as multivariate regression analysis) was performed in the Statistica 28.0 software package (StatSoft). Logarithms of metal content were used in multivariate statistical analysis, considering that the analyzed samples do not correspond to the normal distribution law. The criteria for normal distribution in the sample were the values of asymmetry (less than 1) and kurtosis (less than 5) [31].
Distribution of metals in bottom sediments and water of the Karagayly River was assessed using the multiplicative index (MC), which was calculated as the product of the content (bottom sediments in %, water in mg/l) of Cu, Zn and Cd, multiplied by 1000 to reduce the digit value.
Results and discussion
The system of geochemical barriers that formed on the river before 2011 mainly consisted of acidic-alkaline barriers. Besides that, in wide and shallow river sections, covered with reeds and sedges, complex barriers of mechanical and biogeochemical types were formed, but they were not effective at metals immobilization. The first alkaline barrier was formed by mixing of acidic sub-dump drainage water (pH 1-3 [32]) and slightly alkaline groundwater. Today it still works, since water use changes of recent years have not affected this section of the river. The mixing of two water flows leads to the neutralization of acidic sub-dump filtrate and intensive precipitation of whitish powder, consisting of gypsum, clay minerals and sulfates of heavy metals (here and further referred as “aqueous sulfates”). Precipitate is rich in the studied metals. According to the results obtained in different years, Zn content in aqueous sulfates reaches 1.57-1.78 %, Cu – 0.91-1.24 %, Cd – 11.5-13.3 mg/kg; values of the multiplicative indicator are also very high (Fig.2). The pH value of mixed water varies widely – from 4.98 to 7.52. The pH value depends on the rainfall before sampling and the ratio of drainage and groundwater. Besides that, a weak relationship between values of pH and water flow from the dumps was found. Intensive rains and high values of water flow are combined with low pH and vice versa. Despite massive sulfates precipitation, concentration of ore metals in the sub-dump water remains extremely high. During the research period, content of elements in water reached 24.1-111 mg/l (Zn), 0.58-21 (Cu), 0.048-0.39 mg/l (Zn). High concentrations determine maximal values of the multiplicative indicator throughout the entire observation period (Fig.3).
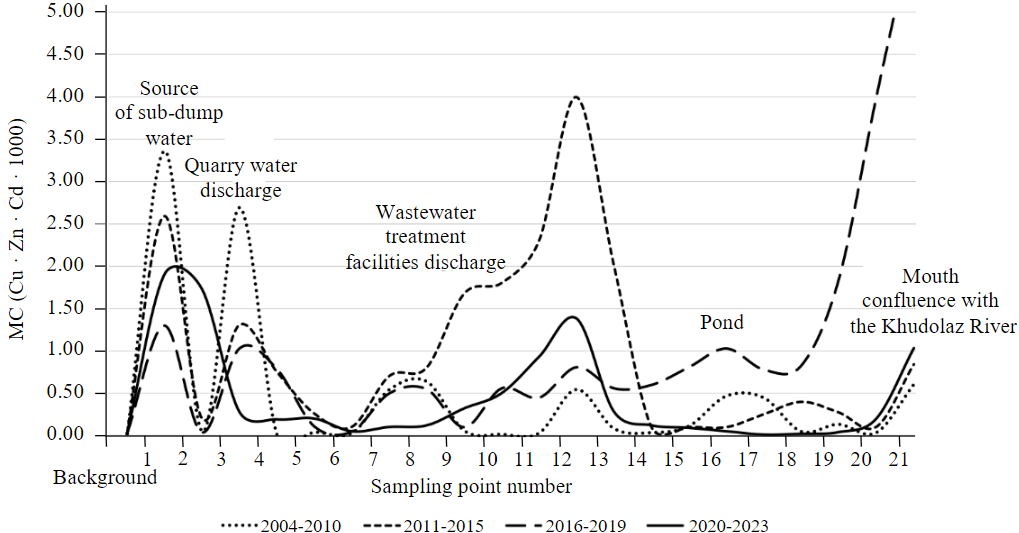
Fig.2. Values of the multiplicative indicator for Karagayly River bottom sediments at different hydrogeochemical stages of the watercourse evolution
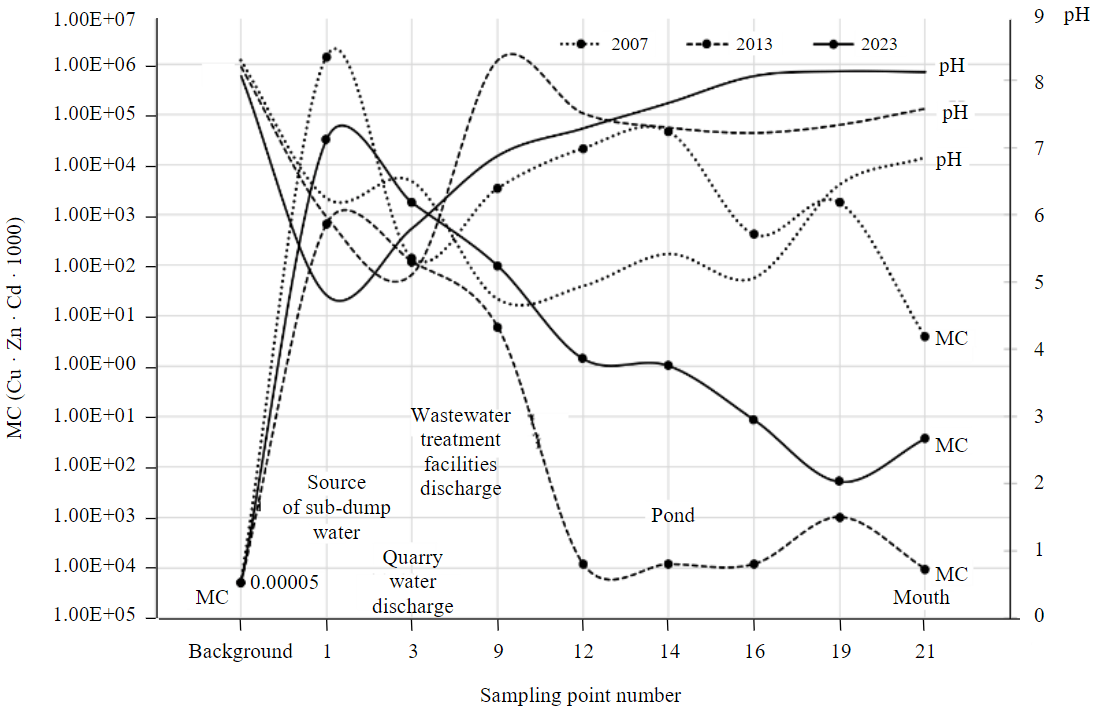
Fig.3. Values of the multiplicative indicator in the Karagayly River water at different hydrogeochemical stages of the watercourse evolution
Quantitative assessment of metal deposition efficiency for the alkaline barrier is difficult, since sampling of sub-dump water before mixing with groundwater is impossible. However, characteristics of drainage water leaving dumps are available [32]: Zn concentration reaches 100 g/l, Cd – 0,38 g/l. These values suggest a decrease of metals concentration by three orders of magnitude. Such change probably occurs due to dilution, but one should not underestimate the role of the alkaline barrier in the sub-dump “lake”, where melanterites and, possibly, chalcanthite and goslarite are formed [32]. The last two minerals crystallize due to the supersaturation of solution with Cu and Zn. In the current hydrogeochemical situation, acidic drainage is diluted and an alkaline barrier works, reducing the flow of metals into superaquatic and subaquatic sites. The concentration of metals in water leaving the dumps is far from saturation. It is indicated by the absence of detectable own mineral phases of Zn, Cu and Cd in aqueous sulfates [33]. However, a ratio of mobile (sorption-carbonate) species of Zn, Cu, Cd in the precipitate is high – 33, 26 and 22 % of the total content, respectively. Thus, the effectiveness of described acidic-alcaline barrier is relatively high, but it does not provide the required level of water purification (to background levels). Some metals are transported downstream as free cations and in suspended forms.
Until 2019, quarry water was discharged downstream of the Karagayly River. At that point flow of quarry water exceeded the river flow. pH of wastewater was low and stable (3.32-4.95), which ensured high metals concentration in water and prevented precipitation of aqueous sulfates. Metals concentration in wastewater reached 36.6-49 (Zn), 5.4-8.1 (Cu), 0.13-0.15 mg/l (Cd). High flow va-lues of wastewater led to the acidification of neutral and slightly alkaline river waters (pH decreased to 4.60-5.30). So, one more acidic-alkaline barrier was formed, but effectiveness of metals immobilization was low. In alkalized environment metals from the wastewater were partly adsorbed by clay minerals. Some elements’ content in bottom sediments exceeded their content upstream and downstream 4.2 times (Zn, Cd) and 5 times (Cu). However, peaks of the multiplicative indicator at this barrier were consistently lower than in the upper reaches of the river (see Fig.2). River water acidification led to the aqueous sulfates dissolution and further migration of metals in solution. This is reflected by the shape of the multiplicative indicator graph (for water): clear increase (2007) and slow decrease downstream to the point of the river Karagayly mouth (see Fig.3). In turn, a gradual pH increase provoked the repeated formation of aqueous sulfates, visually recognised in the middle and lower reaches of the river (to the Kalinino village). According to the results of metals’ sequential extraction from bottom sediments samples, the percentage of the sulfate forms in the upper reaches and near the river mouth was 78 % and 15 % of total content (Cu), 29 and 9 % (Zn), 43 and 7 % (Cd) respectively [30]. The existed system of barriers in the considered hydrogeochemical environment did not ensure decrease of metals’ concentrations to background levels at the confluence of the Karagayly and the Khudolaz Rivers (Fig.3).
The treatment facilities, launched in 2011, were focused on removal of chalcophilic elements from the quarry and mine water coming from the Kamagan quarry. Liming followed by flocculation were used; no preliminary Fe removal was carried out. So, alkaline water (pH > 10), purified from chalcophilic metals, but rich in Fe2+, was being discharged into the river. As a result, an artificial acidic-alkaline and sorption (hydroxide) barrier appeared. After the treatment facilities launch, the river water pH at the discharge site increased from 4.5-4.8 to 7.5-9.1. This led to decrease of metals’ stability in the solution. Precipitation of aqueous sulfates intensified. Even more significant was the iron bicarbonate formation FeSO4 + Ca(HCO3)2 ® CaSO4 + Fe(HCO3)2 with its transition to hydroxide: 2Fe(HCO3)3 ® 2Fe(OH)3¯ + 6CO2 [30], accompanied by adsorption and almost total precipitation of Zn, Cu and Cd. It provided a decrease of metals concentration in river water to background levels (Fig.3).
The results of bottom sediments’ column study confirmed the sedimentation acceleration (the upper layer was formed after the treatment facilities launch). This part of the river is wide, shallow and calm. Under these conditions, sedimentation rate increased to several centimeters per year (approximately). The metals’ content in the upper layer of sediments was significantly higher than in the lower one: 10 times (Zn – 19200 and 1580 mg/kg in the upper and lower layers respectively), 1.3 times (Cu, 8260 and 6380 mg/kg), 20 times (Cd, 48.2 and 2.4 mg/kg). This feature is reflected by the multiplicative indicators graph (Fig.2). Even more significant changes were found for river water. The comparison series is based on data from 2007 and 2013. Below the point of wastewater discharge, Zn content decreased from 21 to 0.098 mg/l, Cu – from 1.6 to 0.042 mg/l and Cd – from 0.1 to 0.001 mg/l. Thus, it can be stated that the purification level of river water at the formed complex barrier was around 97 % for Cu, and more than 99 % for Zn and Cd. Before and after treatment facilities’ operating period, this part of the river lacked a geochemical barrier and was a transition one, not affecting elements’ migration (Fig.3).
Dredging works without the necessary scientific justification led to removal and disturbance of sediments, accumulated during the development of the ore deposit. The ditch-like shape of the channel increased water flow rate and banks erosion. Fe2+, once deposited in the sediments, began to move with water flow, oxidize and precipitate not only in Karagayly, but also in the Khudolaz River at a distance of more than 25 km downstream [33]. In 2016-2019 sediments accumulated at the bottom of the river contained 20-25 % Fe. During dredging and straightening of the riverbed, all geochemical barriers in the middle and lower reaches of the Karagayly River were destroyed. An increase of the water flow energy led to the metals’ transit and accumulation at the river mouth (Fig.3). At the same time, emerging of additional pollution source (erosion of technogenic sediments) caused a slight increase of the multiplicative indicator values (in comparison with other periods of the river’s evolution; see Fig.2). In the river mouth one more alkaline barrier appeared, causing massive precipitation of Fe oxyhydroxides and metals sorbed by them.
The current stage of water use is characterized by cease of quarry water discharge, stop of water acidification, and the decrease in the metals supply in the upper reaches of the Karagayly. As a result, river water pH rapidly returned to background values (from 4.8 to 8.13), metals concentrations began to follow a pattern of steady decrease along the river (towards the mouth) and almost reached background values (Zn – 0.50, Cu – 0.073, Cd – 0.001 mg/l; Fig. 3). The values of multiplicative indicator in bottom sediments are low in the middle and lower reaches of the river (see Fig.2). Described features of the Zn, Cu and Cd distribution in water and bottom sediments are caused by significant decrease in the metal's influx (after cease of the quarry water discharge). However, erosion of sediments, oxidation and migration of elements, previously deposited in sediments are possible.
Analysis of nature-like technologies effectiveness for treating polluted waters is based on study of the bottom sediments’ composition (they can be considered as sewage sludge – SS). The reason is that the assessment of sediments’ toxicity and the aquatic environment’s secondary pollution risk during SS’s removal and neutralization are necessary. Bottom sediments of a watercourse formed on different types of geochemical barriers can be taken as model objects. Not only total content of metals, but also mobile forms’ content and elements’ forms (using sequential extraction method) were studied (2007, 2022). Considering that in the lower reaches of the river limestone is present, the mobile forms extracted by the ammonium acetate buffer solution should be considered as the sum of exchangeable, adsorbed on clay minerals and contained in carbonates species. In the context of the Karagayly River hydrogeochemical conditions changing (with significant variability in the incoming volumes of technogenic material and contrasting acidic-alkaline conditions), an assessment of the metals’ total content and their mobile forms were carried out for each stage of the river transformation (Table 1). However, only data for the middle and lower reaches of the river were used for calculations, because in the upper reaches the hydrogeochemical situation did not change until 2019.
Table 1
Total content (mg/kg) and ratio of mobile forms (%) of metals in bottom sediments of the Karagayly River (middle and lower reaches)
|
Stages, years, number of samples |
Cu |
Zn |
Cd |
Fe |
Mn |
|
2004-2010, n = 24 |
4786±751* 15 |
5200±888 36 |
9.3±1.8 38 |
122800±16500 0.4 |
824±88 9.3 |
|
2011-2015, n = 10 |
7186±1314 31 |
8928±1465 49 |
16.3±4.1 47 |
101200±16500 4.5 |
1410±324 18 |
|
2016-2019, n = 12 |
8522±1839 9 |
11207±4257 34 |
22.4±8.5 20 |
214000±18600 0.5 |
943±223 7.3 |
|
2020-2023, n = 12 |
4013±2582 27 |
5752±1638 45 |
11.3±3.0 75 |
97100±11600 2.3 |
1090±91 49 |
* In the numerator – mean ± standard error of the mean (p = 0.05), in the denominator – percentage of mobile forms.
One-factor analysis of variance of the metal content in bottom sediments allowed to identify the following features (caused by changes in the water use system). Sediments accumulated in 2011-2015 and 2016-2019 (during the operating period of the treatment facilities) stand out for significantly higher metals content in comparison to ones formed before 2011 and after 2019. It is caused by the influence of the alkaline Fe-rich water discharge on the immobilization of chalcophiles. Until 2011, the transit regime with the removal of metals into the Khudolaz River prevailed. Moreover, in 2016, as a result of dredging work, masses of metals from the exposed sediments were involved in the migration. After the cease of the quarry water discharge and the treatment facilities closure, the transit regime was restored, but masses of elements involved were significantly reduced.
At the same time, the ratio of metals main species was changed. The maximal ratio of mobile forms is typical for periods with consistently high pH values (2011-2015 and 2020-2023), which provide favorable conditions for the accumulation of metals, including in the adsorbed state. The transit regime (2004-2010) determined lower content and ratio of mobile forms in bottom sediments. It contributed to the removal of metals to higher-order rivers. During dredging and mobilization of large masses of ferrous iron, the ratio of exchangeable forms decreased due to the increased accumulation by Fe hydroxides. At the same time, Mn activity did not increase due to its low content in sediments. The contrast in the Fe and Mn content is caused by different pH values of these elements’ hydroxides precipitation.
The geochemical specialization of the forming bottom sediments at each of the four identified hydrogeochemical stages (determined by the water use system) is confirmed by the results of discriminant analysis of the metal content in sediments, performed by stepwise selection using the Wilks method. As in the previous calculation of average values, only samples taken in the middle and lower reaches of the river were taken into account (starting from the area affected by wastewater treatment facilities). The dependent variable in the analysis was the four distinguishable stages. The discriminating variables were Cd, Fe, Mn, Pb, Ni, Co content, as well as the pH value. According to the results, 89.1 % of the grouped observations from the original 55 were classified correctly, which highlights the reliability of the geochemical specialization of the hydrogeochemical environment types. The greatest contribution to the classification of groups was made by the pH level and the content of Fe and Co in bottom sediments.
During the treatment facilities’s operating period (2011-2019) and after the cease of quarry water discharge, the ratio of mobile forms decreased. It is related to the formation of a hydroxide sorption barrier and the adsorption of metals on newly formed Fe and Mn hydroxides. This is confirmed by the results of sequential extraction performed in 2007 [30] and 2022. At the first stage the share of hydroxide (reducible) forms was 1-7 %, then in 2022 it increased to 6-15 %, with Zn having the maximum affinity for the reducible form. The importance of hydroxide-sorption accumulation of metals is proven by the results of microscopic analysis: despite the absence of their own secondary mineral phases in sediments, Cu, Zn, Cd are found in the formations of microcrystalline aggregates with Fe compounds and clay minerals, acting as adsorbents (Fig.4, a). Mn-Zn aggregates were discovered (Fig.4, b) together with apatite and Fe oxides [33].
High content and mobility of metals in sediments caused their significant toxicity, which all chalcophilic elements (including Pb, As, Hg, Sb, etc.) contribute to. It was proven by the biotesting of bottom sediments (seven samples) using Daphnia magna Straus and the Chlorella vulgaris Beijer. A 100 % death of Daphnia in almost all samples was observed, with the exception of the river mouth zone, where it decreased to 80 %. The deviation of optical density from the Chlorella culture control for three samples was 50-80 %, for the remaining four samples it was more than 80 %. The results confirm contemporary ideas about emergent pollutants (such as As, Mn, xanthates) entering the environment during the development of sulfide ore deposits and their high toxicity in highly mine-ralized, sulfate-rich water [34].
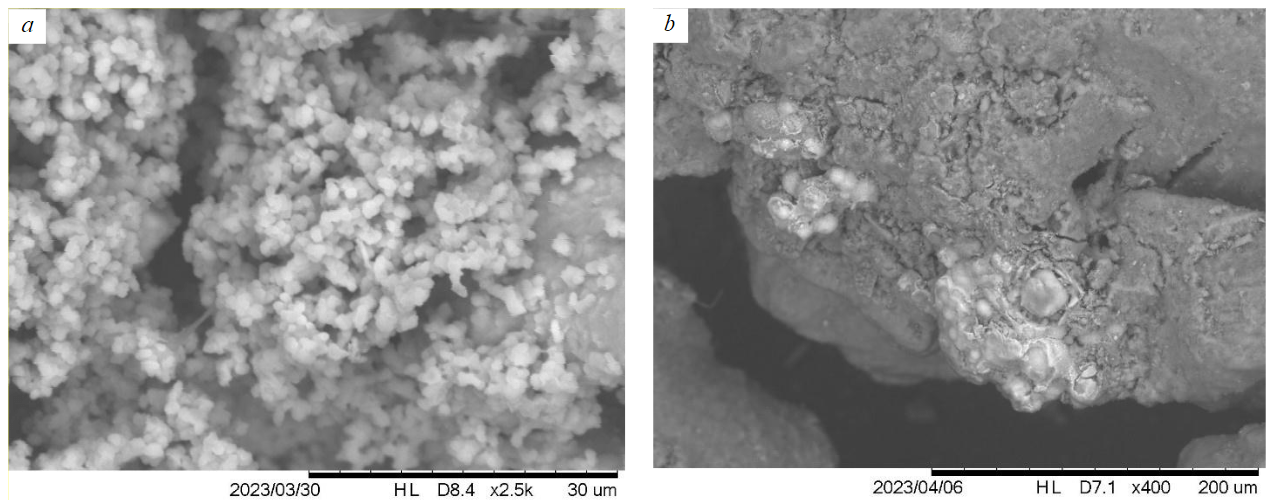
Fig.4. Mineral phases of iron in bottom sediments of the Karagayly River near the dumps of the Sibay quarry: a – iron hydroxides (in the area of acid quarry water discharge); b – ferromanganese formations (light shade) with Zn, Cu and Co against the background of a clay matrix (dark shade)
Statistical modeling of the metal deposition mechanisms at different hydrogeochemical stages of watercourse evolution was carried out using multiple regression analysis (MRA). The hypothesis is the possibility of phase transitions and precipitation of metals in an acidic environment (low pH of river water) via adsorption by newly formed Fe and Mn hydroxides, clay minerals and organic matter. Hydroxides, clay minerals and Corg can act as metal carrier phases. Thus, when conducting MRA, Cu, Zn and Cd content in bottom sediments successively acted as dependent variables. The content of Fe, Mn, Corg, pelitic (clay minerals), silt (secondary mineral phases) fractions and water pH were consi-dered as predictors.
Regression equations were obtained (p = 0.05). Main mechanisms and factors of metal deposition at the artificial barriers of the Karagayly River were revealed (Table 2). Phase transitions and accumulation of Cu are caused by adsorption on Fe hydroxides, an increase in pH, and the Mn content in sediments (in descending order of correlation significance). The coefficient of determination is 0.385, which explains 38.5 % of the variance of Cu content in sediments. In this case, Fe plays a decisive role, according to the value b = 0.472 (Table 2). Mn has a negative coefficient value in the regression equation and a negative correlation with the dependent variable. It can be considered as a factor counteracting Cu accumulation. To explain this phenomenon, one should take into account alkalization of the Karagayly water downstream, (the pH of Mn hydroxides precipitation is significantly higher than that of Fe and Cu hydroxides). Mn accumulation occurs in the upper and lower reaches, and Cu accumulation in the middle reaches, which corresponds to the precipitation regime of Fe hydroxides (Table 3).
Table 2
The role of carrying phases and physicochemical conditions in the deposition of Zn, Cu, Cd into bottom sediments of the Karagayly River according to the results of multiple regression analysis
|
Dependent variable |
Model (n = 57) |
Determination coefficient |
Standardized coefficient b |
Regression equation |
|
Cu |
Fe |
0.385 |
0.472 |
Cu = 0.044 + 0.59 Fe + 2.264 pH – 0.392 Mn |
|
pH |
0.383 |
|||
|
Mn |
–0.250 |
|||
|
Zn |
pH |
0.330 |
0.406 |
Zn = 1.434 + 1.882 pH + 0.271 Mn |
|
Mn |
0.245 |
|||
|
Cd |
pH |
0.352 |
0.476 |
Cd = –4.718 + 3.081 pH + 0.614 Fe |
|
Fe |
0.400 |
Table 3
Total content (mg/kg) of metals in bottom sediments of the upper, middle and lower reaches of the Karagayly River
|
Reach of the river,number of samples |
Cu |
Zn |
Cd |
Fe |
Mn |
|
Upper, n = 24 |
6649 ± 1778* |
10053 ± 2203 |
9.92 ± 1.53 |
59100* ± 18400 |
1400 ± 250 |
|
Middle, n = 10 |
6420 ± 468 |
5780* ± 563 |
9.54 ± 1.18 |
142600 ± 10200 |
940 ± 93 |
|
Lower, n = 12 |
4326* ± 713 |
7970 ± 1150 |
16.2 ± 2.47 |
115300 ± 16600 |
1280 ± 128 |
* Mean ± standard error of the mean (p = 0.05) – significantly low content (according to the results of analysis of variance).
The activity of the Zn transition into the solid phase is determined by the pH value and Mn content (in order of their significance). Dependence on these predictors explains 33 % of the variance. Thus, there are two main mechanisms of Zn accumulation: precipitation in sulfates (in case of pH increase) and adsorption on newly formed Mn hydroxides. The former (b = 0.41) dominates over the latter (b = 0.245). Similar pH values of Mn and Zn hydroxides precipitation and the coinciding peak of concentrations in the lower reaches of the river support the hypothesis of adsorption on newly formed Mn hydroxides as opposed to interaction with Fe hydroxides (Table 3).
Cd accumulation is caused by the precipitation with sulfates along with water alkalization and adsorption by Fe hydroxides. These mechanisms accounted for 35.2 % of the variance. The values of the pH and Fe standardized coefficients are close (see Table 2). The peak concentration, like that of Zn, is observed in the lower reaches, which is explained by the high pH of the Cd hydroxide precipitation, Cd stability in solution, and a single source of influx (oxidation of sphalerite).
Intensity of ore elements water migration in secondary dispersion halos of pyrite copper-zinc ores: Cd (1.3) – Zn (1.0) – Cu (0.7) – Fe (0.04) confirms the revealed patterns of metals and their potential carrying phases migration and accumulation [32].
Preliminary analysis of the Corg adsorption and complexing activity (based on MRA) establishes its inclusion as a predictor in the regression equation for Zn content. It confirms that research of organic matter as precipitating agent at copper-zinc-pyrite ore development sites is promising. However, decisive conclusions require higher number of objects (in this research 19 samples were studied).
When determining the main mechanisms of metal precipitation, the role of carbonates was not taken into account, due to absence of quantitative data. However, in the bottom sediments of the Karagayly River lower reaches, an increase in the number of carbonate phases is noted. It is caused by the bedrock: Karamalytash complex (D2kr) mafic igneous rocks is replaced with the Kizil complex (C1-2kz) sedimentary rocks. The latter has predominantly carbonate composition. It is reflected by increase of carbonate forms according to sequential extraction in 2007 (in the first hydrogeochemical stage of the evolution of a watercourse with low pH values and a high content of metals in water). The ratio of carbonate species of metals increased from the source to the mouth of the river: Cu – 9.2-51, Zn – 24.5-36.7, Cd – 8.1-31.7 %. In 2022 (the fourth stage of evolution) the ratio of carbonate forms of metals noticeably decreased, which could be caused by a decrease of elements’ concentrations in river water.
A high ratio of oxidizable (sulfate) forms (Cu up to 86, Zn up to 29, Cd up to 60 % of total content) in sediments in 2007 should be mentioned, as well as an increase in ratio of the reducible (connected with Fe-hydroxides) form in 2022: Cu – up to 43, Zn – up to 31 and Cd – up to 24 %. The obtained results are fully consistent with the model of sedimentation and its transformation during the change in hydrogeochemical conditions from the acidic-alkaline type to the hydroxide-sorption type.
Conclusion
Long-term studies of metals migration and accumulation conditions (during the development of the Sibay copper-zinc-pyrite deposit) provide arguments of effective immobilization and sedimentation of pollutants by geochemical barriers (natural, technogenic and artificial). Changes in the management of quarry and dump drainage water of the deposit along the Karagayly River caused formation of different hydrogeochemical conditions. Each stage of water use has specific geochemical features of water and bottom sediments composition, as well as ore metals’ migration and accumulation, which was confirmed using multivariate statistics. Over a long period, acidic-alkaline technogenic barriers were the main factors of river water purification and pollutants’ precipitation. Their effectiveness in regard to specific metals is controlled by pH values. Precipitation occurs in sulfate form when water is alkalized. Complex alkaline-hydroxide-sorption barrier was formed unintentionally after the launch of wastewater treatment facilities which dumped into the Karagayly River highly alkaline Fe-rich water. This barrier turned out to be more effective, removing 97 % of Cu and more than 99 % of Zn and Cd.
Statistical modeling revealed the behavior of the studied metals in different hydrogeochemical environments, as well as factors and mechanisms of their accumulation. Different metals have distinct characteristics of phase transitions. Cu precipitation occurs due to adsorption by newly formed Fe hydroxides, and, to a lesser extent, with sulfates depositing as water pH increasing. Revealed antagonism with Mn hydroxides is probably caused by different physicochemical conditions of precipitation.
Zn accumulation mechanisms are different. They are mainly related to phase transition in sulfates (as pH increases) and include formation of zinc’s own mineral phases like goslarite. The second mechanism is adsorption by newly formed Mn hydroxides, which corresponds to the idea of similar conditions for the precipitation of metal hydroxides. Cd behavior reflects conditions intermediate between these of Cu and Zn. Contribution of both mechanisms (related to Fe hydroxides and aqueous sulfates) is equal. Antagonism to Mn is absent (unlike the case of Cu). Organizing purification systems, one must take into account distinct behavior of Fe and Mn in contrasting physicochemical conditions arising during sulfide ores development. It is caused by significantly different pH values of these metals’ hydroxides precipitation.
Study of metals’ forms in bottom sediments and preliminary calculations indicate potential role of organic matter (Zn) and carbonates (Cu, Zn) in metals’ accumulation. However, confirmation requires additional research using representative sampling. Analysis of clay minerals participation in precipitation processes did not give a positive result.
Assessment of geochemical barriers effectiveness in wastewater purification allows to conclude that using of nature-like technologies in situ (in watercourses, canals and other water drainage systems of developed pyrite deposits) without complex treatment facilities is promising. Developed statistical models are preliminary but still can be used for needs of experimental studies and engineering.
References
- Aqib Zahoor, Guozhu Mao, Xinming Jia et al. Global research progress on mining wastewater treatment: a bibliometric analysis. Environmental Science: Advances. 2022. Vol. 1. Iss. 2, p. 92-109. DOI: 10.1039/D2VA00002D
- Castellar J.A.C., Torrens A., Buttiglieri G. et al. Nature-based solutions coupled with advanced technologies: An opportunity for decentralized water reuse in cities. Journal of Cleaner Production. 2022. Vol. 340. N 130660. DOI: 10.1016/j.jclepro.2022.130660
- Wolkersdorfer C. Mine Water Treatment – Active and Passive Methods. Berlin: Springer, 2022, p. 328. DOI: 10.1007/978-3-662-65770-6
- Udachin V.N., Aminov P.G., Filippova K.A. Geochemistry of the Southern Urals mining technogenesis. Ekaterinburg: UrO RAN, 2014, p. 251 (in Russian).
- Chanturiya V., Masloboev V., Makarov D. et al. Geochemical barriers for environment protection and recovery of nonferrous metals. Journal of Environmental Science and Health. Part A. 2014. Vоl. 49. Iss. 12, p. 1409-1415. DOI: 10.1080/10934529.2014.928543
- Denisova Yu.L. Scientific justification for the use of artificial geochemical barriers based on mining waste for wastewater treatment and extraction of non-ferrous metals: Avtoref. dis. … kand. tekhn. nauk. Moscow: Institut problem promyshlennoi ekologii Severa, 2018, p. 21 (in Russian).
- Nazarov A.M., Latypova F.M., Araslanova L.Kh. et al. Research of efficiency of natural and modified sorbents for purification of industrial sewage from heavy metal ions. Nanotechnologies in Construction. 2018. Vol. 10. N 5, p. 125-143 (in Russian). DOI: 10.15828/2075-8545-2018-10-5-125-143
- Nikashina V.A. Permeable reactive barriers as a way to protect the environment from pollutions. Natural sorbents for solving environmental problems. Mathematical modeling and calculation of processes. Review. Sorption and Chromatography Processes. 2019. Vol. 19. N 3, p. 289-304 (in Russian). DOI: 10.17308/sorpchrom.2019.19/746
- Savenko A.V. Experimental modeling of the immobilization of heavy metals at the carbonate adsorption-precipitation geo-chemical barrier. Geochemistry International. 2016. Vol. 54. N 8, p. 719-731. DOI: 10.1134/S0016702916060069
- Torres E., Lozano A., Macías F. et al. Passive elimination of sulfate and metals from acid mine drainage using combined lime-stone and barium carbonate systems. Journal of Cleaner Production. 2018. Vol. 182, p. 114-123. DOI: 10.1016/j.jclepro.2018.01.224
- Limper D., Fellinger G.P., Ekolu S.O. Evaluation and microanalytical study of ZVI/scoria zeolite mixtures for treating acid mine drainage using reactive barriers – Removal mechanisms. Journal of Environmental Chemical Engineering. 2018. Vol. 6. Iss. 5, p. 6184-6193. DOI: 10.1016/j.jece.2018.08.064
- Mayacela-Rojas C.M., Molinari A., Cortina J.L. et al. Removal of Transition Metals from Contaminated Aquifers by PRB Technology: Performance Comparison among Reactive Materials. International Journal of Environmental Research and Public Health. 2021. Vol. 18. Iss. 11. N 6075. DOI: 10.3390/ijerph18116075
- Kremenetskaya I.P., Mazukhina S.I., Drogobuzhskaya S.V., Ivanova T.K. Physical and chemical modeling of the ZnSO4-CaO(MgO)-SiO2-H2O system. Transactions of the Kola Science Centre of RAS. Series: Natural Sciences and Humanities. 2022. Vol. 1. N 2, p. 93-99 (in Russian). DOI: 10.37614/2949-1185.2022.1.2.011
- Mei Li, Yan Kang, Haoqin Ma et al. Efficient removal of heavy metals from aqueous solutions using Mn-doped FeOOH: Performance and mechanisms. Environmental Research. 2023. Vol. 231. Part 1. N 116161. DOI: 10.1016/j.envres.2023.116161
- Ahmed M., Elektorowicz M., Hasan S.W. GO, SiO2, and SnO2 nanomaterials as highly efficient adsorbents for Zn2+ from industrial wastewater – A second stage treatment to electrically enhanced membrane bioreactor. Journal of Water Process Engineering. 2019. Vol. 31. N 100815. DOI: 10.1016/j.jwpe.2019.100815
- Chanturiya V.A., Masloboev V.A., Suvorova O.V. et al. Justification of technologies for processing and reducing the environmental danger of waste from mining enterprises: the main results and prospects of scientific cooperation. Transactions of the Kola Science Centre of RAS. Series: Natural Sciences and Humanities. 2022. Vol. 1. N 2, p. 9-19 (in Russian). DOI: 10.37614/2949-1185.2022.1.2.002
- García-Valero A., Martínez-Martínez S., Faz A. et al. Environmentally sustainable acid mine drainage remediation: Use of natural alkaline material. Journal of Water Process Engineering. 2020. Vol. 33. N 101064. DOI: 10.1016/j.jwpe.2019.101064
- Kharko P.A., Nureev R.R., Pashkevich M.A. Possibility of using limestone-based geochemical barriers for purification of waste water from metals. The Eurasian Scientific Journal. 2020. Vol. 12. N 6, p. 9 (in Russian).
- Smirnov Iu.D., Kharko P.A., Pashkevich M.A. Patent N 2779420 RU. Method for purification of wastewater from iron and copper ions. Publ. 06.09.2022. Bul. N 25 (in Russian).
- Bayurova Yu.L., Nesterov D.P., Korneva E.A. et al. Artificial geochemical barriers for solving environmental and technological problems. Vestnik MGTU. 2013. Vol. 16. N 3, p. 536-541 (in Russian).
- Cheremisina O.V. Aspect of technology protection of hydrosphere against ions of heavy metals in a zone of influence of objects. Journal of Mining Institute. 2013. Vol. 203, p. 116-119 (in Russian).
- Petrakova E.A. Macrophytes in phytoremediation and bioindication of water: Avtoref. dis. … kand. biol. nauk. Bryansk: Bryanskii gosudarstvennyi universitet im. akademika I.G.Petrovskogo, 2017, p. 23 (in Russian).
- Mudruňka J., Matunová Kavková K., Kučerová R. et al. Technology of the Biological Treatment of Mine Water at the Kohinoor II Mine. Engineering Proceedings. 2023. Vol. 57. Iss. 1. N 34. DOI: 10.3390/engproc2023057034
- Petrov D.S., Korotaeva A.E., Pashkevich M.A., Chukaeva M.A. Assessment of heavy metal accumulation potential of aquatic plants for bioindication and bioremediation of aquatic environment. Environmental Monitoring and Assessment. 2023. Vol. 195. Iss. 1. N 122. DOI: 10.1007/s10661-022-10750-0
- Kapahi M., Sachdeva S. Bioremediation Options for Heavy Metal Pollution. Journal of Health and Pollution. 2019. Vol. 9. Iss. 24. N 191203. DOI: 10.5696/2156-9614-9.24.191203
- Khalid A., Khan Y., Hadi R. et al. Bioremediation of heavy metals from wastewater through soil bacteria. Journal of Xi’an Shiyou University, Natural Sciences Edition. 2023. Vol. 66. Iss. 04, p. 15. DOI: 10.17605/OSF.IO/CV3MU
- Prajapati A.V., Baxi N.N., Dave S.R., Tipre D.R. Mycosorption: a sustainable approach for removing heavy metals from simulated polluted water in non-competitive and competitive systems. Environment, Development and Sustainability. 2024, p. 19. DOI: 10.1007/s10668-024-04524-6
- Kosarev A.M., Vladimirov A.G., Khanchuk A.I. et al. Devonian-Carboniferous magmatism and metallogeny in the South Ural accretionary-collisional system. Geodynamics & Tectonophysics. 2021. Vol. 12. N 2. С. 365-391. DOI: 10.5800/GT-2021-12-2-0529
- Maslennikov V.V., Ayupova N.R., Maslennikova S.P., Tseluyko A.S. Hydrothermal biomorphoses of massive sulfide de-posits: biomineralization, trace elements, and bio-productivity criteria. Yekaterinburg: UB RAS, 2016, p. 388 (in Russian).
- Opekunov A.Y., Opekunova M.G., Somov V.V. et al. Influence of the exploitation of Sibay deposit (the Southern Urals) on the transformation of metal migration in subordinate landscapes. Moscow University Bulletin. Series 5. Geography. 2018. N 1, p. 14-24 (in Russian).
- Nasledov A. IBM SPSS Statistics 20 and AMOS: professional statistical data analysis. St. Petersburg: Piter, 2013, p. 416 (in Russian).
- Emlin E.F. Technogenesis of the Urals pyrite deposits: Avtoref. dis. … d-ra geol.-mineral. nauk. Sverdlovsk: Sverdlovskii gornyi institut imeni V.V.Vakhrusheva, 1988, p. 30 (in Russian).
- Opekunov A., Janson S., Opekunova M. Hydrogeochemical transformation of small rivers under the impact of mining production (on the example of the Karagayly river, Sibay). Steppe Science. 2022. N 3, p. 12-22 (in Russian). DOI: 10.24412/2712-8628-2022-3-12-22
- Ryskie S., Neculita C.M., Rosa E. et al. Active Treatment of Contaminants of Emerging Concern in Cold Mine Water Using Advanced Oxidation and Membrane-Related Processes: A Review. Minerals. 2021. Vol. 11. Iss. 3. N 259. DOI: 10.3390/min11030259
