Experimental simulation of a system of swamp biogeocenoses to improve the efficiency of quarry water treatment
- 1 — Ph.D., Dr.Sci. Head of the Department of Geoecology Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 2 — Postgraduate Student Saint Petersburg Mining University ▪ Orcid
- 3 — Ph.D. Director of the Scientific Center Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
Abstract
Mining activities were producing large quantities of wastewater contaminated with nitrogen compounds and metals. With insufficient treatment, these pollutants are released into the environment and have a toxic effect on living organisms. Constructed wetlands are now widely adopted as wastewater treatment systems because of the combination of physical, chemical and biological processes for the removal of contaminants. In this study, an experimental system was modeled to improve the efficiency of the quarry wastewater treatment of a mining enterprise by sharing the higher aquatic vegetation: broad-leaved cattail (Typha latifolia L.), common water-plantain (Alisma plantago-aquatica L.), jointed rush (Juncus articulatus L.) and lower aquatic vegetation (Chlorella sp.). Concentrations of nitrogen compounds and metal were analyzed both in the model and in the treated solution of quarry wastewater for calculation of treatment efficiency. Concentrations of the pollutants in the tissues of the higher aquatic vegetation were analyzed to assess the accumulation capacity and efficiency of translocation of the pollutants. The results of the experimental study showed the practical applicability of the constructed integrated treatment system to reduce the concentration of pollutants in quarry wastewater, as well as increasing the efficiency of treatment by introducing lower aquatic vegetation into the system
Introduction
Quarry wastewater from mining activities is characterized by a multicomponent composition [1, 2]. Biogenic compounds and heavy metals, being a major pollutant in wastewater and entering surface and groundwater streams, cause significant disturbances in the natural processes of ecosystems [3, 4]. Due to the effect of biomagnification, heavy metals accumulate in the food chain and eventually enter human and living organisms, causing disruption of vital systems [5, 6]. Nutrient inputs lead to eutrophication [7], which leads to reduced dissolved oxygen and light in natural reservoirs [8].
Constructed wetlands are becoming an economical alternative to traditional wastewater treatment methods. They are systems that absorb pollutants from natural ecosystem processes, including coastal aquatic vegetation, microorganisms and bacteria [9]. Currently, constructed wetlands are used to treat wastewater from agriculture [7, 10], livestock [11], as well as mining [12], oil and gas [13, 14] and metallurgical industries [15].
There are four types of constructed wetlands: free-water surface, horizontal and vertical subsurface flow, hybrid systems [16]. Depending on the oxygen situation created by the wastewater flow mode [17], many pollutants are effectively removed: nitrogen compounds (NH4+, NO2–, NO3–) [18, 19], phosphorus (Н2РО4–, НРО42–, РО43–) [20, 21], hydrolysable metals (Al, Fe, Mn) [8, 22], and other metals and metalloids that are an integral part of the ore, as well as host and waste rock (Cr, Cu, Mo, Ni, Pb, Zn, As, etc.) [23-25].
All components of the constructed wetlands participate in wastewater treatment. There is a growing interest in the technology to reduce the concentration of pollutants due to vegetation and associated microorganisms, named “phytoremediation” [22]. Depending on the pollutant input mechanism in plant tissues, phytoremediation is divided into several constituents [26]. For example, in the treatment of heavy metal contaminated wastewater, rhizophilization plays an important role, i.e. absorption of pollutants by the root system, and as a result of the phytoextraction process, the input and accumulation of pollutants in plant tissues takes place.
The purpose of the study was to assess the applicability of the system of constructed wetlands for the treatment of quarry wastewater of a mining enterprise. The study analyzed the effectiveness of reducing the concentration of pollutants in the model solution of quarry wastewater by an integrated system consisting of the higher aquatic vegetation of broad-leaved cattail (Typha latifolia L.), common water-plantain (Alisma plantago-aquatica L.), jointed rush (Juncus articulatus L.) and lower aquatic vegetation (Chlorella microalga). In the experimental study, model solution and vegetation samples were taken for further analysis of the concentration of nitrogen compounds and metals.
Methods
The study was carried out based on data from a coal mining enterprise located in the territory of the Republic of Khakassia, one of the oldest mining areas of Siberia (Fig.1). The climate of the area is sharply continental and characterized by frosty winter and warm summer. The temperature range in summer is from 9.8 to 25.2 °С with an average monthly temperature of 16.8 °С.
Fig.1. The location of the enterprise
Mining of a coal deposit is carried out in an open way. Ammonium nitrate-based mixtures are used as explosives for drilling and blasting operations. Quarry wastewater results from the discharge of groundwater aquifers and from the entry of precipitation into quarries. Based on hydrological characteristics, four aquifers have been identified in the area of the quarry field, the waters of which belong to the bicarbonate, sulfate-bicarbonate, chloride-bicarbonate or sulfate-chloride type.
To date, the volume of quarry water is about 15,000 m3/day, with the share of groundwater is about 70 %. To drain the resulting water from the pit sump, pumps are used, pumping quarry wastewater into the storage pond. During the winter months, the water inflow declines, wastewater accumulates and is not treated because of the low volume.
Based on the materials obtained from the studied coal mining enterprise, the qualitative and quantitative composition of the model solution of quarry wastewater was determined. To prepare the model solutions the following salts were used: sodium nitrate, sodium nitrite, calcium chloride, iron sulphate (II) 7-aqueous, potassium sulfate, magnesium chloride 6-aqueous, sodium hydrocarbonate, sodium chloride and potassium 1-substituted phosphate. In addition, an aqueous solution of the main microcomponents necessary for the vital activity of plants, namely Mn, B, Mo, Cu, Zn, Co, the concentrations of which were taken equal concentrations in the quarry wastewater of the enterprise, was used. The aliquot of the prepared solutions was selected based on the total volume of the initial model solution, which was delivered to the experimental setup for treatment in one cleaning cycle. Table 1 shows the composition of the model solution.
Table 1
Qualitative and quantitative composition of model solution
|
Pollutant |
Concentration of pollutant in solution, mg/dm3 |
Maximum permissible concentration*, mg/dm3 |
|
Nitrate |
100 |
45 |
|
Nitrite |
6.0 |
3.0 |
|
Iron |
0.04 |
0.3 |
|
Calcium |
76.8 |
– |
|
Sodium + Potassium |
404 |
– |
|
Hydrocarbonate |
656.8 |
– |
|
Phosphate |
0.16 |
3.5 |
|
Manganese |
0.011 |
0.1 |
|
Boron |
0.004 |
0.5 |
|
Molybdenum |
0.001 |
0.07 |
|
Copper |
0.01 |
1.0 |
|
Zinc |
0.001 |
5.0 |
|
Cobalt |
0.0004 |
0.1 |
|
рН |
7-8 |
– |
* SanRiN 1.2.3685-21 “Hygienic standards and requirements to ensure safety and (or) harmlessness of environmental factors for humans”.
The experimental setup was modelled after the free-water surface constructed wetlands (Fig.2). The choice of this system was justified by the lowest capital and operating costs for implementation into the existing treatment system, namely the operating storage pond of the enterprise. The setup consisted of wetland vegetation (4), loading for its rooting (6), microorganisms and a glass tank (5), in the volume of which the treatment took place. The experimental setup was equipped with a full-spectrum lamp (3) to simulate 10-hour daylight. The plastic container with a volume of 60 litres (1) preceded the experimental setup as a distribution tank of the model solution of quarry wastewater. The metal container of 65 litres (8) was used as a receiving tank for the treated model solution. To achieve better treatment efficiency, a species of Chlorella microalgae (7) was introduced as the dominant form of the microorganism in the tank of the experimental setup. A cell suspension with an optical density of 0.275 was added in the amount of 5 % of the total unit volume (5).
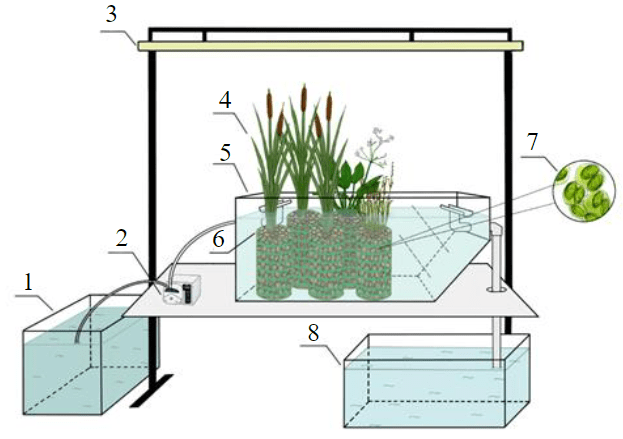
Fig.2. Scheme of the experimental setup
As a wetland vegetation the experimental setup used broad-leaved cattail (Typha latifolia L.), common water-plantain (Alisma plantago-aquatica L.) and jointed rush (Juncus articulatus L.), which are characterized by high accumulation of pollutants from wastewater [27-29]. These plant species were selected in natural growing conditions and acclimatized within two weeks at the experimental site. The acclimatized vegetation specimens were divided into two identical groups, one of which was not involved in the experimental study, so that the concentrations of pollutants in plant tissues were assumed as background. The second group of vegetation was placed in the experimental setup in a percentage ratio of 60:20:20 in constructed plastic mesh containers with a diameter of 15 cm and a height of 25 cm. A mixture of crushed stone and expanded clay by 5-20 and 10-20 mm, respectively, was used as a loading.
The species ratio was determined according to the characteristics of the vegetation in question and the capacity of the experimental setup. Broad-leaved cattail was preferred as the main type in the treatment system due to its higher phytoremediation potential. Specimens of common water-plantain and jointed rush were placed in the experimental setup as auxiliary species for intensification of the treatment process, as well as increasing the surface of fixing the dominant species of microorganism due to the developed high specific area of the root system surface.
The model solution from the container (1) was continuously fed into the experimental setup by means of a peristaltic pump (2). The solution dosing rate was 8.6 ml/min and was calculated from the average hydraulic time of 3-4 days for the treatment system at the enterprise. The experimental study was conducted on the basis of the Scientific Center “Ecosystem” of the Saint Petersburg Mining University at a temperature 23 ± 0.5 °C and a relative humidity of 84 ± 2 %.
The model solution of the quarry wastewater was sampled at three points: at the inlet and outlet of the experimental constructed wetland, and directly from the volume of treated water in the setup. A total of six consecutive cleaning cycles of the model solution were completed within 26 days. Each sample was placed in a 50 ml sterile plastic tube, marked and cooled to 4 °C before analysis.
To control the acidity of the model solution, pH values were determined using the Expert-001-3 ionometer. Nitrate and nitrite were measured by liquid chromatography on the LC-20 Shimadzu device. The metal concentration in the solution was determined by an atomic-emission spectrometer with inductively coupled plasma ICPE-9000 (Shimadzu).
To assess the concentration of pollutants in plant tissues, at the end of the experimental study, plant specimens of each species were selected, the total biomass of which provided a reliable analysis. The selected plant specimens were subjected to the main stages of preparation: they were divided into the main vegetative organs – leaves and roots, dried to an air-dry condition and ground using a laboratory blender Waring LB20EG.
To determine the nitrate concentration, 1 g of each pre-prepared plant sample was placed in a flask containing 50 ml of 1 % aluminum potassium sulfate solution. The resultant solutions were then mixed on a magnetic stirrer for 3 min, after which they were filtered to produce a transparent filtrate used in the measurement. The concentration of nitrate was determined by the ionometric method by Expert-001-3. The obtained nitrate mass concentrations in the aqueous extract were recalculated in milligrams per kilogram of dry mass.
Heavy metal concentrations in plants tissues were analyzed in several stages. The pre-prepared plant samples were ashed in the Nabertherm LT 15/11 muffle furnace at 550 °C for 10 h. The metals were extracted from the ash to the solution by acid extraction with the addition of 33 % HNO3. The metal concentration in the solution was determined by an atomic-emission spectrometer with inductively coupled plasma ICPE-9000 (Shimadzu).
The obtained values of mass concentration of metals in the solution were converted to dry mass. For this purpose, ash values were additionally determined: 1 g of each pre-prepared plant sample was placed in the crucible and further into the Nabertherm LT 15/11 muffle furnace, where the samples were held at 550 °С before the visible traces of carbon compounds disappeared. The calculation of ash content was carried out according to GOST P 56881-2016.
To assess the influence of metals and plant species on the concentrations of accumulated metals in plant tissues, a two-factor analysis of ANOVA was conducted. The significance level in the calculation was assumed to be 0.05. The Pearson linear correlation coefficient was calculated to estimate the transport of pollutants in plant tissues. The statistical processing of the results of the experimental study was carried out using the MS Excel software product.
Results discussion
The introduction of Chlorella microalgae into the wastewater treatment system has a number of advantages. During growth, microalgae may absorb pollutants, mainly nitrogen and phosphorus, from wastewater [30, 31]. The viability of microalgae is due to the fact that the model solution of quarry wastewater contains biogenic compounds as well as heavy metals, which are essential macro- and micro-components, making it as close as possible to the composition of the nutrient medium, necessary for microalga growth. In addition, Chlorella microalgae has high photosynthetic activity and biomass growth rate [32]. Oxygen production rate is 2-10 О2/m2×h and depends on ambient light, reaching maximum values at noon [33-35].
In the experimental setup, an aerobic environment was established during the study due to photosynthetic oxygen release by vegetation and microalgae. In many constructed wetlands, less oxygen produced during the day is consumed at night [30], increasing dissolved oxygen concentration in the water. In the current situation, nitrite oxidation occurred during the nitrification process [19] and by the end of the 4th cleaning cycle – a steady decrease of concentration in the model solution by 99 %. Because nitrite was converted to nitrate, there was no significant change in nitrate concentration at the outlet of the experimental setup.
When comparing the treatment efficiencies of nitrite in the model solution of quarry wastewater and in mine waters of similar composition exclusively with the help of higher aquatic vegetation [12] an increase of disposal efficiency of 18 % was observed in the current study. This shows that the introduction of Chlorella microalgae into the treatment system has contributed to the intensification of the process of absorbing pollutants from the quarry wastewater.
Nitrate and ammonium forms of nitrogen are known to undergo a step-by-step transformation as part of nitrogen exchange when they enter the plant cell. The absorbed ammonium is directly involved into the synthesis of amino acids and amides. At the same time, nitrate is initially reduced to nitrite form in the cytosol of the cell and further in the chloroplast – to ammonium, which is also supplied for amino acid and amide synthesis. An excess nitrate, not used for biosynthesis, is stored in the vacuole cell [36]. Therefore, in order to quantify the accumulated nitrogen in plant tissues, nitrate concentration was analyzed in the experimental study (Fig.3).
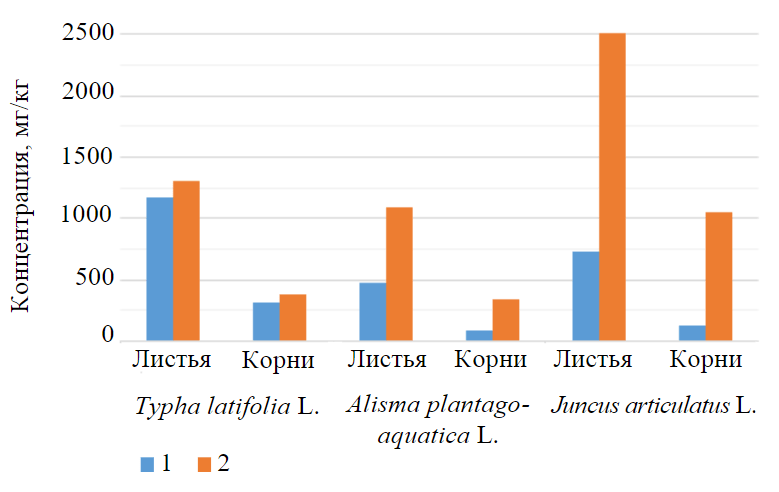
Fig.3. Nitrate concentration in different plant tissues 1 – background values; 2 – experimental
As can be seen, the intensity of nitrogen accumulation in different plant species differs due to the physiology and morphology of each species. However, there was a greater concentration of nitrate in leaves than in roots for the plant species studied. In the leaves of broad-leaved cattail, common water-plantain and jointed rush, nitrate concentrations at the end of the experimental study were 3.4, 3.2 and 2.4 times higher than those of roots. The highest values of accumulated pollutant in leaves and roots are jointed rush – 2,514.2 and 1,056.1 mg/kg, respectively.
In all tissues of the studied vegetation, at the end of the experimental study, nitrate concentrations increased significantly compared to background values. The greatest increase in the nitrate concentration in the leaves compared to the background values was observed in the jointed rush (3.4 times), followed by the common water-plantain (2.3 times) and broad-leaved cattail (1.1 times). Nitrate accumulation in vegetation roots has a similar sequence of species and increases relative to background values of 8.3, 3.7 and 1.2 respectively.
The data obtained allow to speak about the effective accumulation of nitrate in the tissues of the studied vegetation and, as a consequence, the absorption of nitrogen compounds from the aqueous solution. It should be noted that the absorption efficiency of these compounds by vegetation is influenced by the ratio of N/P in the aquatic environment. The phosphorus concentration in the model solution of quarry wastewater (0.05 mg/dm3) is several tens of times lower than the nitrogen compound concentration. Nevertheless, the obtained concentrations of accumulated nitrate in plant tissues suggest that nitrogen compounds can be removed at a low phosphorus level, which is consistent with the study [29].
Due to the well-established aerobic environment, hydrolysable metals were effectively removed from the model solution in the experimental setup. The mean concentration of Mn during the experimental study in the solution at the inlet of the setup was 0.011 mg/dm3 and at the outlet 0.0016 mg/dm3. There has been an average decrease in Mn concentration of 85.5 % since the first cleaning cycle. Iron removal was less effective at 12.9 %.
The accumulation of metals in vegetation, like nitrate, is determined by its physiology and morphology. The results of the study show high concentrations of accumulated metals in broad-leaved cattail (Fig.4).
Compared to background concentrations of Mo, Zn, Cu, Mn and Fe, there was a significant increase in concentrations, namely 1.7; 2.7; 3.3; 1.9 and 2.5 times, respectively. The highest accumulative capacity was found for iron (1,889 mg/kg) and manganese (1,211 mg/kg). The following series of metal accumulations is observed: Fe > Mn > Zn > Cu > Mo, which is consistent with the data obtained in previous studies of the accumulative capacity of this species [37]. There was no significant increase in metal concentrations in the tissues of other studied plant species in the study, but all plant tissues exhibited identical accumulations.
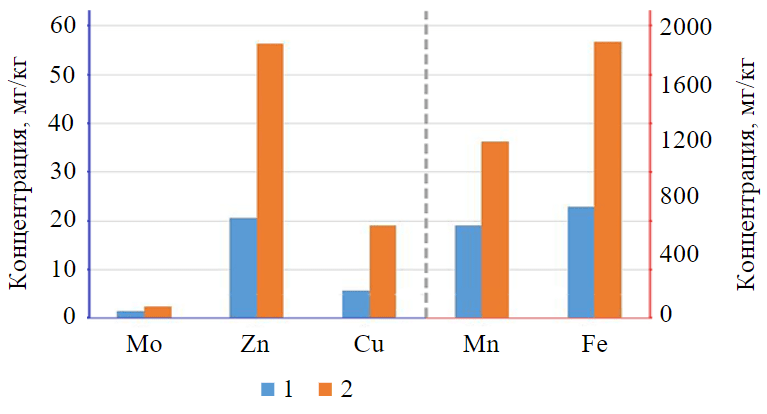
Fig.4. The concentration of metals in the leaves of broad-leaved cattail (Typha latifolia L.) (the values of the columns to the left of the dotted line refer to the blue vertical axis, to the right – to the red) 1 – background values; 2 – experimental
It should be noted that vegetation’s ability to absorb metals depends on its type and metal [12]. Some plant species, being hyperaccumulators, tolerate the exceedance of concentrations of pollutants in aquatic systems and, as a consequence, their high content in their biomass [23, 38]. The conducted study found a positive correlation between the concentration of pollutants in plant tissues and metals (p < 0.007). This factor accounted for 43.3 %, which is sufficient considering all factors influencing the uptake of metals by vegetation, environmental characteristics (pH, light, temperature, salinity, etc.), and plant species (in this study, factor share – 11.4 %) [39].
Once absorbed by vegetation roots, metals can accumulate directly in the roots and (or) be transported to the aboveground organs. In order to assess the transport efficiency of the pollutants in the studied vegetation, the correlation Pearson analysis between metal concentrations in the aboveground and underground organs was carried out. Uniform transport of absorbed metals to the aboveground organs is carried out through the xylene vessels in the form of chelate complexes immobilized in the intracellular spaces of the root [40], which is indirectly confirmed by the obtained directly proportional dependence (r = 0.88, 0.99, 0.99 for broad-leaved cattail, common water-plantain and jointed rush respectively).
The effective transfer of metals from underground to aboveground organs is indicated by the ratio of metal concentration in shoots to roots greater than one [41]. In the experimental study, this value was shown by broad-leaved cattail to Zn, Cu and Mn, as well as common water-plantain to Zn and Cu (Table 2).
Table 2
Concentration of metals in plant tissues at the end of the experimental study
|
Plant species |
Concentration, mg/kg |
Multiplicity |
||||||
|
Zn |
Cu |
Mn |
Fe |
Zn |
Cu |
Mn |
Fe |
|
|
Broad-leaved cattail |
|
|
|
|
3.5 |
2.6 |
18.7 |
0.8 |
|
Leaves |
56.3 |
19.0 |
1,211.1 |
1,889 |
||||
|
Roots |
15.9 |
7.2 |
64.6 |
2,436 |
||||
|
Common water-plantain |
|
|
|
|
1.5 |
1.1 |
0.9 |
0.2 |
|
Leaves |
18.3 |
8.4 |
123.6 |
567 |
||||
|
Roots |
11.9 |
7.7 |
131.4 |
2,300 |
||||
|
Jointed rush |
|
|
|
|
0.5 |
0.2 |
0.3 |
0.1 |
|
Leaves |
19.0 |
9.9 |
73.2 |
1,046 |
||||
|
Roots |
39.9 |
47.7 |
229.9 |
9,105 |
||||

Fig.5. Development of root system of broad-leaved cattail (Typha latifolia L.)
All three plant species used in the experimental study are characterized by a developed root system with a large surface area, which has a positive effect on the rate of absorption of the pollutants from the solution [29]. To visualize the results, the development of the root vegetation system is given by the example of broad-leaved cattail (Fig.5).
At the beginning of the experimental study, the suppression of the root system of planted vegetation was observed in terms of discolouration and disturbance of root growth. At the end of the vegetation adaptation period to the conditions of the experimental setup, after two weeks from the beginning of the experimental study, a slight growth of young roots in each species was found (Fig.5, a). During the initial absorption period, there was an increase in size and growth of new roots (Fig.5, b). Already at the beginning of the sustained uptake period there was active branching of the roots (Fig.5, c).
The root system was placed at a distance of 10 cm from the bottom of the constructed plastic mesh containers. By the end of the experimental study, the roots were growing through loading to the bottom of the containers. There was an increase in the area of the root system by an average of 85 % of the original planted one, which allows for almost complete recovery and, as a result, of successful adaptation of the vegetation to the concentrations of pollutants in the model solution. All plant species studied during the experimental study showed growth of both underground and above-ground parts.
Conclusion
In the result of the study practical applicability of a complex method of biological treatment was proved, following the example of a system of free water surface constructed wetlands for coal-mining enterprise conditions. Based on the data obtained, it can be concluded that the treatment system in question can be applied to mining enterprises with a similar chemical composition of the wastewater produced.
In addition to the ability to self-clean this system through natural processes, the efficiency of pollutant removal is enhanced by the combined use of higher and lower aquatic vegetation. The plant species used have successfully adapted to the model solution of quarry wastewater, as evidenced by the developing root system and the growth of the aboveground part.
The conditions created by the experimental study contributed to the active removal of nitrite and hydrolysable metals from the model solution. In order to increase the efficiency of nitrate nitrogen removal from quarry wastewater, it is recommended that anaerobic zones be established in constructed wetlands to intensify the denitrification process.
All plant species (broad-leaved cattail, common water-plantain and jointed rush) have demonstrated the ability to accumulate nitrate in their tissues, with the predominant concentration noted in the above-ground part. The greatest accumulations were observed in the leaves and roots of the jointed rush – 2,514.2 and 1,056.1 mg/kg, respectively.
In relation to metals (Mo, Zn, Cu, Mn and Fe), broad-leaved cattail showed the highest accumulation ability. There was an active accumulation of metals in the leaves of vegetation, mainly Fe and Mn. In addition, active transport of Zn, Cu, Mn and Zn and Cu and Cu was detected in broad-leaved cattail and common water-plantain, which is one of the characteristics of hyperaccumulator plants.
References
- Chukaeva M.A., Povarov V.G., Sverchkov I.P. Iron-Containing Metalworking Wastes as a Chemosorbent for Wastewater Treatment from Molybdenum Ions. Moscow University Chemistry Bulletin. 2020. Vol. 75. Iss.1, p. 36-42. DOI: 10.3103/S0027131420010058
- Letuyev K.V., Kovshov S.V., Gridina E.B. The Technology of Hydrodedusting of Coal Pits’ Auto Roads Using Purified Wastewater and Drainage Water. Ecology and Industry of Russia. 2020. Vol. 24. N 1, p. 30-33 (in Russian). DOI: 10.18412/1816-0395-2020-1-30-33
- Litvinova T.E., Suchkov D.V. Comprehensive approach to the utilisation of technogenic waste from the mineral resource complex. Mining Informational and Analytical Bulletin. 2022. № 6-1, p. 331-348 (in Russian). DOI: 10.25018/0236_1493_2022_61_0_331
- Chukaeva M.A., Petrov D.S. Assessment and analysis of metal bioaccumulation in freshwater gastropods of urban river habitats, Saint Petersburg (Russia). Environmental Science and Pollution Research. 2023. Vol. 30. Iss. 30, p. 7162-7172. DOI: 10.1007/s11356-022-21955-8
- Alekseenko V.A., Shvydkaya N.V., Bech J. et al. Trace element accumulation by soils and plants in the North Caucasian geochemical province. Journal of Mining Institute. 2021. Vol. 247, p. 141-153 (in Russian). DOI: 10.31897/PMI.2021.1.15
- Sergeev V.V., Cheremisina O.V., Fedorov A.T. et al. Interaction features of sodium oleate and oxyethylated phosphoric acid esters with the apatite surface. ACS Omega. 2022. Vol. 7. N 3, p. 3016-3023. DOI: 10.1021/acsomega.1c06047
- Nizam N.U.M., Hanafiah M.M., Noor I.M., Karim H.I.A. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Applied Sciences. 2020. Vol. 10. Iss. 8. N 2712. DOI: 10.3390/APP10082712
- Dhir B. Phytoremediation: Role of Aquatic Plants in Environmental Clean-Up. India: Springer India, 2013, p. 111. DOI: 10.1007/978-81-322-1307-9
- Choudhury M.I., Segersten J., Hellman M. et al. Importance of plant species for nitrogen removal using constructed floating wetlands in a cold climate. Ecological Engineering. 2019. Vol. 138, p. 126-132. DOI: 10.1016/j.ecoleng.2019.07.012
- Kosolapova S.M., Smal M.S., Rudko V.A. et al. A new approach for synthesizing fatty acid esters from linoleic-type vegetable oil. Processes. 2023. Vol. 11. N 5. N 1534. DOI: 10.3390/pr11051534
- Wu S., Kuschk P., Brix H. et al. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review. Water Research. 2014. Vol. 57, p. 40-55. DOI: 10.1016/j.watres.2014.03.020
- Etteieb S., Zolfaghari M., Magdouli S. et al. Performance of constructed wetland for selenium, nutrient and heavy metals removal from mine effluents. Chemosphere. 2021. Vol. 281. N 130921. DOI: 10.1016/j.chemosphere.2021.130921
- Shammazov I.A., Batyrov A.M., Sidorkin D.I., Van Nguyen T. Study of the Effect of Cutting Frozen Soils on the Supports of Above-Ground Trunk Pipelines. Applied Sciences. 2023. Vol. 13. N 3139. DOI: 10.3390/app13053139
- Pavlinery N., Skoulikidis N.Th., Tsihrintzis V.A. Constructed Floating Wetlands: A review of research, design, operation and management aspects, and data meta-analysis. Chemical Engineering Journal. 2017. Vol. 308, p. 1120-1132. DOI: 10.1016/j.cej.2016.09.140
- Kataki S., Chatterjee S., Vairale M.G. et al. Constructed wetland, an eco-technology for wastewater treatment: A review on types of wastewater treated and components of the technology (macrophyte, biolfilm and substrate). Journal of Environmental Mana-gement. 2021. Vol. 283. N 111986. DOI: 10.1016/j.jenvman.2021.111986
- Rozema E.R., VanderZaag A.C., Wood J.D. et al. Constructed Wetlands for Agricultural Wastewater Treatment in Northeastern North America: A review. Water. 2016. Vol. 8. N 173. DOI: 10.3390/w8050173
- Vymazal J., Březinová T. Accumulation of heavy metals in aboveground biomass of Phragmites australis in horizontal flow constructed wetlands for wastewater treatment: A review. Chemical Engineering Journal. 2016. Vol. 290, p. 232-242. DOI: 10.1016/j.cej.2015.12.108
- Zhang Lingling, Sun Zhenzhong, Xie Jia et al. Nutrient removal, biomass accumulation and nitrogen-transformation functional gene response to different nitrogen forms in enhanced floating treatment wetlands. Ecological Engineering. 2018. Vol. 112, p. 21-25. DOI: 10.1016/j.ecoleng.2017.12.021
- Fernandez-Fernandez M.I., de la Vega P.T.M., Jaramillo-Morán M.A., Garrido M. Hybrid Constructed Wetland to Improve Organic Matter and Nutrient Remova. Water. 2020. Vol. 12. N 2023. DOI: 10.3390/w12072023
- White S.A. Plant Nutrient Uptake in Full-Scale Floating Treatment Wetlands in a Florida Stormwater Pond: 2016-2020. Water. 2021. Vol. 13. N 569. DOI: 10.3390/w13040569
- Sandoval L., Zamora-Castro S.A., Vidal-Álvarez M., Marín-Muñiz J.L. Role of Wetland Plants and Use of Ornamental Flowering Plants in Constructed Wetlands for Wastewater Treatment: A review. Applied Sciences. 2019. Vol. 9. N 685. DOI: 10.3390/app9040685
- Sabreena, Hassan S., Bhat S.A. et al. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants. 2022. Vol. 11. N 1255. DOI: 10.3390/plants11091255
- Chamba-Eras I., Griffith D.M., Kalinhoff C. et al. Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon. Plants. 2022. Vol. 11. N 1186. DOI: 10.3390/plants11091186
- Golik V.I., Marinin M.A. Practice of underground leaching of uranium in blocks. Mining Informational and Analytical Bulletin. 2022. N 6-1, p. 5-20 (in Russian). DOI: 10.25018/0236_1493_2022_61_0_5
- Zubkova O., Alexeev A., Polyanskiy A. et al. Complex Processing of Saponite Waste from a Diamond-Mining Enterprise. Applied Sciences. 2021. Vol. 11. N 6615. DOI: 10.3390/app11146615
- Sladkovska T., Wolski K., Bujak H. et al. A Review of Research on the Use of Selected Grass Species in Removal of Heavy Metals. Agronomy. 2022. Vol. 12. N 2587. DOI: 10.3390/agronomy12102587
- Khan S., Ahmad I., Shah M.T. et al. Use of constructed wetland for the removal of heavy metals from industrial wastewater. Journal of Environmental Management. 2009. Vol. 90, p. 3451-3457. DOI: 10.1016/j.jenvman.2009.05.026
- Kataki S., Chatterjee S., Vairale M.G. et al. Constructed wetland, an eco-technology for wastewater treatment: A review on types of wastewater treated and components of the technology (macrophyte, biolfilm and substrate). Journal of Environmental Management. 2021. Vol. 283. N 111986. DOI: 10.1016/j.jenvman.2021.111986
- Wang Kunlun, Hu Qian, Wei Yumin et al. Uptake Kinetics of NH4+, NO3− and H2PO4− by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn. Sustainability. 2021. Vol. 13, p. 434. DOI: 10.3390/su13010434
- Reddy K.R., DeLaune R.D., Inglett P.W. Biogeochemistry of Wetlands: Science and Applications. Boca Raton: CRC Press, 2022, p. 734. DOI: 10.1201/9780429155833
- Gonçalves A.L., Pires J.C.M., Simões M. A review on the use of microalgal consortia for wastewater treatment. Algal Research. 2017. Vol. 24, p. 403-415. DOI: 10.1016/j.algal.2016.11.008
- Randrianarison G., Ashraf M.A. Microalgae Plant (Chlorella sp.) for Wastewater Treatment and Energy Production. Ekoloji. 2018. Vol. 27. Iss.106, p. 1455-1465.
- Rearte T.A., Celis-Plá P.S.M., Neori A. et al. Photosynthetic performance of Chlorella vulgaris R117 mass culture is moderated by diurnal oxygen gradients in an outdoor thin layer cascade. Algal Research. 2021. Vol. 54. N 102176. DOI: 10.1016/j.algal.2020.102176
- Doucha J., Straka F., Lívanský K. Utilization of flue gas for cultivation of microalgae (Chlorella sp.) in an outdoor open thin-layer photobioreactor. Journal of Applied Phycology. 2005. Vol. 17, p. 403-412. DOI: 10.1007/s10811-005-8701-7
- Doucha J., Lívanský K. Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. Journal of Applied Phycology. 2006. Vol. 18, p. 811-826. DOI: 10.1007/s10811-006-9100-4
- Pearsall W.H. Nitrogen metabolism in plants: methods and protocols. New York: Humana Press, 2020, p. 178. DOI: 10.1007/978-1-4939-9790-9
- Petrov D.S., Korotaeva A.E., Pashkevich M.A., Chukaeva M.A. Assessment of heavy metal accumulation potential of aquatic plants for bioindication and bioremediation of aquatic environment. Environmental Monitoring and Assessment. 2023. Vol. 195. N 122. DOI: 10.1007/s10661-022-10750-0
- Wan Shuming, Pang Jun, Li Yiwei et al. Hydroponic Phytoremediation of Ni, Co, and Pb by Iris Sibirica L. Sustainability. 2021. Vol. 13. N 9400. DOI: 10.3390/su13169400
- Olguín E.J., Sánchez-Galván G. Phytofiltration of Heavy Metals: Assessment of the Key Factors Involved in the Design of a Sustainable Process. Comprehensive Biotechnology. 2011. Vol. 6, p. 207-213. DOI: 10.1016/B978-0-08-088504-9.00379-2
- Rezania S., Taib S.M., Md Din M.F. et al. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. Journal of Hazardous Materials. 2016. Vol. 318, p. 587-599. DOI: 10.1016/j.jhazmat.2016.07.053
- Yan An, Wang Yamin, Tan Swee Ngin et al. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Frontiers in Plant Science. 2020. Vol. 11, p. 1-15. DOI: 10.3389/fpls.2020.00359
