Flotation separation of titanite concentrate from apatite-nepheline-titanite ores of anomalous zones of the Khibiny deposits
Abstract
Titanium raw materials are widely used for the synthesis of various functional materials – sorbents of radionuclides and rare earth elements, various additives, filler pigments, etc. Since most of titanium concentrates are imported, in line with the import substitution program, production of titanite concentrate from apatite-nepheline ores of the Khibiny deposits is a promising trend for supplying national industry with titanium raw materials. The article presents the results of laboratory studies of flotation separation of titanite concentrate from apatite-nepheline-titanite ores extracted from the upper ore horizon of the Koashvinskoye deposit, where titanite-enriched ores are concentrated. Recovery of titanite concentrate was accomplished using two reagent modes – a mixture of alkyl hydroxamic and carboxylic acids with the addition of distilled tall oil and a mixture of tall oils with the addition of polyalkyl benzene sulfonic acids. The results of the research showed that the first flotation mode, which allows a selective recovery of titanite into the concentrate (titanite content in the concentrate was 93.5 %) is the most efficient. It was shown that flotation separation of titanite concentrate is preferable compared to the chemical method based on sulfuric acid leaching.
Introduction
Complex apatite-nepheline ores of the Khibiny deposits are a source for obtaining several valuable products. One of them, titanite concentrate, is considered as an alternative replacement for the traditional titanium raw materials in the synthesis of various functional materials – sorbents of radionuclides and rare earth elements [1-3], additives to polymer membranes [4], bipolar plates [5] and electrodes [6, 7] in fuel cells, catalysts [8], filler pigments for accelerating the hydration rate [9], changing the microstructure [10] and physical and mechanical properties of cement [11], etc.
Russia has large own balance reserves of titanium-containing ores; however, this mineral raw material is mainly extracted as a by-product [12, 13], most of titanium concentrates are imported. In line with the import substitution program, production of titanite concentrate from ores of the Khibiny deposits is a promising trend for supplying national industry with titanium raw materials. Mineral titanite is a calcium titanosilicate (CaTiSiO5) containing about 40 % TiO2. It is known that from the current [14, 15] and stored wastes of processing apatite-nepheline ores [16], it is possible to recover titanite concentrate with 28-34 % TiO2 content applying the flotation-magnetic technology. Studies have shown that such titanite concentrate can be used to produce sorbents [17, 18], tanning agents and sealants [19, 20], composite electrode materials [21], and other inorganic materials that are in demand on the Russian market [22].
Titanite content in apatite-nepheline ores of the Khibiny deposits is generally low, amounting to 3-5 %. An exception is the Partomchorr deposit, the upper layers of which are apatite-nepheline-titanite ores with titanite content of 15-17 % [23]. In addition, there are areas at the deposit with an abnormally high mineral content, the so-called “titanite housing” extending for 5 km and to 50 m thick, where titanite content varies from 30 to 80 %. A peculiar occurrence of this type of ores allows considering a possibility of selective extraction and separate processing of ore rich in titanite [22, 23]. Direct acid leaching without preconcentration by physical methods was proposed as a method for beneficiation of such ores with a high content of titanite [24]. The method included successive dissolution of apatite and nepheline from finely ground ore (–0.04 mm) with sulfuric or hydrochloric acid. Thus, from a sample of apatite-nepheline-titanite ore taken from the apical part of the Koashvinskoye deposit, titanite-aegirine products containing about 80 % titanite, 28-32 % TiO2 were obtained using sulfuric acid leaching [22, 25].
However, it is not possible to consider such a method in terms of its applicability on a large-tonnage scale. Fine grinding of ore to the required particle size less than 40 µm, a long-term contact with acid (from 1 to 4 h), use of an aggressive acidic environment are the reasons that limit the use of this beneficiation method by the laboratory scale. Beneficiation by conventional physical methods remains the most attractive procedure for obtaining titanite concentrate compared to chemical cleaning.
This paper presents the results of flotation concentration of apatite-nepheline-titanite ore using two reagent modes: hydroxamic acids and polyalkyl benzene sulfonic acids with addition of tall oils.
Methods
Studies were carried out on the material of apatite-nepheline-titanite ore from the upper horizon of the Koashvinskoye deposit, at which the experts of ICT KSC RAS recovered titanite concentrate by acid leaching [22]. To conduct the research, ore was crushed to a fineness of –1.6 mm successively on the laboratory jaw and roller crushers. Grinding of an ore sub-sample weighing 360 g to flotation fineness was carried out in a laboratory ball mill at S:L:B ratio = 1:0.8:6. After grinding, the content of –0.071 mm class in ore was 35.2 %, that of +0.16 mm class – 28.0 %.
Nonfrothing flotation in Halimond tube was performed on a monomineral fraction of titanite isolated from apatite-nepheline-titanite ore and nepheline crushed to –0.16+0.1 mm; flotation pH – 10.1-10.3. Agitation time of the mineral with the regulator (0.1 % solutions of sulfuric acid or NaOH) is 1 min, with the collector – 2 min. Flotation time is 3 min, air flow rate 5.3 ml/min. The assessment of the strength of reagents fixation on titanite was carried out by flotation in a Halimond tube under desorption conditions. After agitation of the mineral with a reagent, 50 % of the liquid phase was replaced with distilled water with addition of NaOH (рН = 10.1-10.3), stirred for another 2 min, and then floated.
Ore flotation was carried out in a laboratory flotation machine in an open cycle on fresh water. The apatite cycle included rougher (RoF), recleaner (ReF) flotation and two cleaning flotation of the froth product of rougher flotation. Liquid glass depressant (LGD) was fed into grinding; pH of apatite flotation was maintained at 9.6-9.8 by adding the required amount of caustic soda to the pulp.
The cycle of titanite flotation included the rougher, recleaner flotation and two cleaning flotation of the RoF froth product. To activate titanite at the stage of titanite flotation, CaCl2 was added to the pulp in the amount of 25 g/t, pH of titanite flotation, equal to 10.3-10.4, was created by adding caustic soda solution.
Chemical analysis of samples was accomplished by spectrophotometric method using a UNICO spectrophotometer (Р2О5, TiO2) by volumetric titration (Fe, Al2O3).
Mineral composition of feed ore and products obtained as a result of its beneficiation – concentrate and tailings – was determined by the X-ray phase method with a D2 PHASER powder X-ray diffractometer manufactured by Bruker AXS GmbH (Germany).
Discussion of results
Content of the main components in apatite-nepheline-titanite ore, wt. %: 22.48 TiO2; 6.42 Al2O3tot; 5.45 Al2O3 ac/sol; 4.59 P2O5; 2.39 Fetot.
Mineral composition of apatite-nepheline-titanite ore, wt. %: 58.54 titanite; 11.25 fluorapatite; 14.01 nepheline; 2.19 pyroxenes; 1.55 amphiboles; 4.58 feldspars; 0.60 cancrinite; 1.14 sodalite; 2.16 natrolite; 0.18 ilmenite; 0.19 magnetite titanian; 0.35 lamprophyllite; 3.26 micas.
In the well-known technology of complex beneficiation of apatite-nepheline ores [26], titanite flotation occurs at the stage of separation of nepheline and dark-coloured minerals, when the main amount of titanite and aegirine passes into the froth product. The reagent regime used at the operating production for the separation of nepheline concentrate is a mixture of pine and deciduous tall oils; flotation is carried out at pH ~11. However, higher efficiency and selectivity in flotation of dark-coloured minerals is recorded when using alkyl-, aryl benzene sulfonates [27] or a regime based on hydroxamic acids as an additive to tall oils [28, 29].
Dependences of floatability of titanite and nepheline with oleic acid (Ol), polyalkyl benzene sulfonic acid (PABSA) and pelargon hydroxamic acid (C8H17CONHOH) on pH (Fig.1) show that the optimum titanite flotation for oleic and pelargon hydroxamic acid lies in the alkaline region at pH = 8.5-9 (Fig.1, a); for nepheline, the region of maximum interaction with oleic acid is at pH = 8-8.5, and for pelargon hydroxamic acid it is shifted towards pH = 6 (Fig.1, b). The maximum efficiency of PABSA action with both minerals lies in the pH~6 region. In the case of nepheline, the concentration of reagents is several times higher than that required for titanite flotation.
Traditionally, flotation separation of dark-coloured minerals and nepheline is carried out in an alkaline environment (pH~11), which is due to both natural pH of crushed ore suspension and pH = 9.8 of apatite flotation at the first stage of beneficiation. In the region of pH > 9.5, there is a difference in the floatability of titanite and nepheline with pelargon hydroxamic acid (Fig.1).
Concentration dependences of floatability of minerals with various reagents are shown in Fig.2. In view of very weak collecting properties of polyalkyl benzene sulfonic acid as a monoreagent in an alkaline environment, this collector was used as an additive to oleic acid (80 % Ol + 20 % PABSA). Flotation was accomplished at pH = 10.1-10.3, since at a higher alkalinity of the environment the yield of the froth product of flotation with all the reagents under consideration was very low.
An increase in the consumption of collectors led to intense foaming, which violated the methodology and the course of the experiment. A high tendency of silicate minerals to hydration [30], especially in an alkaline environment, apparently, determines a low degree of fixation of hydrophobic reagents.
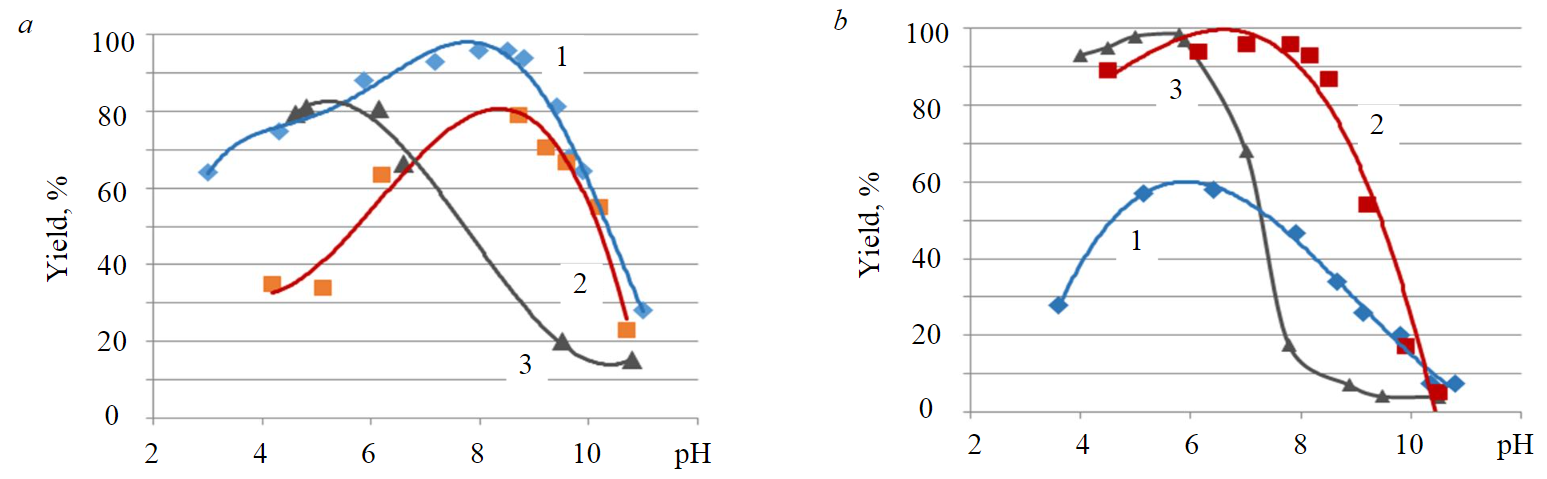
Fig.1. Dependence of titanite (а) and nepheline (b) floatability on рН by different collectors 1 – С8H17CONHOH: С = 0.0001 mol/l (a), С = 0.001 mol/l (b); 2 – Ol: С = 0.00003 mol/l (а), С = 0.00035 (b); 3 – PABSA: С = 0.00003 mol/l (а), С = 0.00007 mol/l (b)
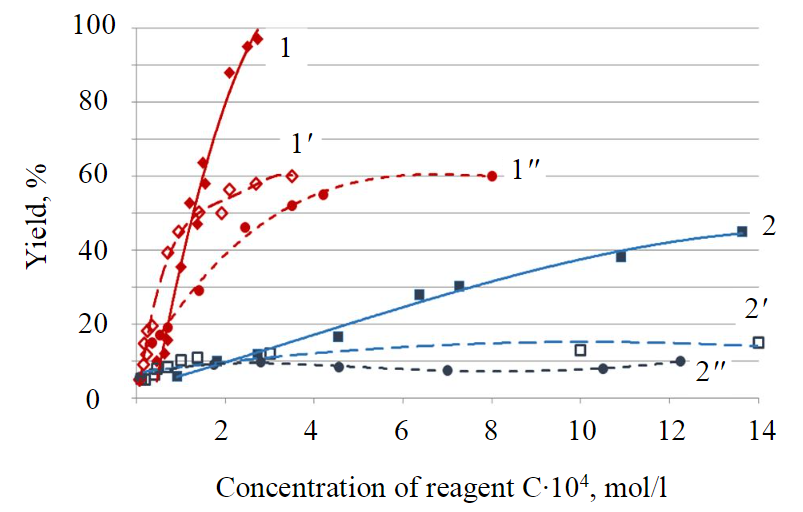
Fig.2. Concentration dependences of floatability of titanite (1, 1', 1'') and nepheline (2, 2', 2'') by collectors С8H17CONHOH (1,2); Ol(1', 2'); Ol+PABSA (1'', 2'')
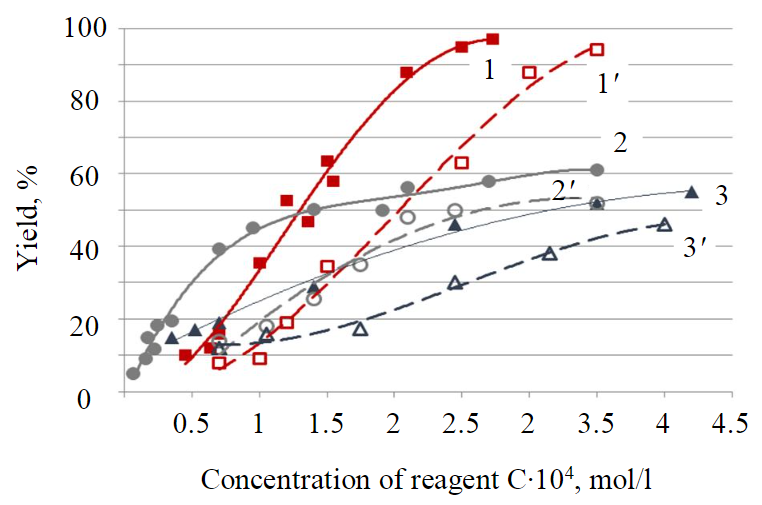
Fig.3. Results of nonfrothing flotation of titanite under normal conditions (1, 2, 3) and desorption conditions (1', 2', 3') using collectors С8H17CONHOH (1); Ol (2); Ol+PABSA (3)
From the results obtained (Fig.2) it follows that the use of pelargon hydroxamic acid allows almost all titanite to be converted into the froth product, while for the regime with oleic acid, the yield of titanite is limited. At the same time, at low concentrations of reagents, a higher hydrophobization of the mineral is achieved, as expected, with using of oleic acid. It is known that at high pH values, insoluble carbonates and hydroxo complexes form on the surface of calcium-containing minerals, which leads to screening of active sites for interaction with the carboxyl collector [31]. Probably, this effect manifests itself during flotation with oleic acid – an increase in reagent concentration does not result in a growing yield of the froth product.
Pelargon hydroxamic acid contains a functional group able of complexing with atoms of transition elements [32], including titanium. Formation of a strong compound with the functional group in case of hydroxamic acid makes the predominant contribution of the functional group to the overall stabilization of the reagent-mineral system and ensures the necessary selectivity and efficiency of this collector, even though the length of the hydrophobic radical in it is much less than in oleic acid.
The strength of reagents fixation on titanite was evaluated from the results of flotation under desorption conditions. A higher hydrocarbon radical of oleic acid and PABSA determines a slightly lower “washing” degree of these reagents at high concentrations. However, the prevalence of pelargon hydroxamic acid is also recorded in this case. Froth product yield of 95 % during flotation under desorption conditions was obtained by increasing the concentration of pelargon hydroxamic acid by ~20 % (Fig.3).
Analysis of mineral composition of feed ore sample (Table 1) showed the need for apatite extraction at the first stage. Previous studies demonstrated that at low content of apatite, it is reasonable to use a mixture of a fatty acid collector (FAC) and polyalkyl benzene sulfonic acids as a collecting mixture (CM). This reagent mode during flotation in an open cycle with rougher, control flotation and two cleaning operations makes it possible to obtain apatite concentrate containing 39.0-38.7 % Р2О5 and reduce its content in the feed of titanite flotation to 0.52-0.61 %. Indicators of the apatite cycle are reproduced in all laboratory experiments with a slight deviation. Table 1 presents an example of the results of apatite flotation in one of them.
For titanite flotation, HA reagent was used, which is a mixture of specially synthesized С7-С8-alkyl hydroxamic and corresponding С7-С8-carboxylic acids in a mass ratio of 3:1. Moreover, acids with different hydrocarbon radicals were taken in equal mass amounts: С7H15CONHOH (C7-HA):C8H17CONHOH (С8-HA) = 1:1 and С7H15COOH:C8H17COOH = 1:1.
Table 1
Indicators of apatite-nepheline-titanite ore beneficiation
|
Flotation product |
Yield, % |
Content, % |
Recovery from ore, % |
||||
|
Р2О5 |
TiO2 |
Al2O3 |
Р2О5 |
TiO2 |
Al2O3 |
||
|
Apatite cycle |
|||||||
|
Reagent regime: 90 g/t Lgl; rougher flotation 100 g/t СM (70 % FAC + 30 % PABSA); |
|||||||
|
Froth product of control flotation |
1.9 |
6.06 |
1.05 |
0.77 |
2.5 |
0.1 |
0.2 |
|
Flotation tail of the I cleaning |
7.6 |
19.64 |
14.15 |
0.39 |
32.4 |
4.8 |
0.5 |
|
Flotation tail of the II recleaning |
3.0 |
37.17 |
2.74 |
0.40 |
24.2 |
0.3 |
0.2 |
|
Apatite concentrate |
3.7 |
38.8 |
0.97 |
0.78 |
31.1 |
0.2 |
0.4 |
|
Apatite flotation tailings (feed of titanium cycle) |
83.8 |
0.54 |
25.34 |
7.60 |
9.8 |
94.6 |
98.7 |
|
Feed |
100 |
4.60 |
22.44 |
6.45 |
100 |
100 |
100 |
|
Titanite cycle |
|||||||
|
Reagent regime 1: 25 g/t CaCl2; rougher flotation 200g/t HA+20g/t DTO; |
|||||||
|
Froth product of control flotation |
9.0 |
0.40 |
6.40 |
21.41 |
0.8 |
2.6 |
29.9 |
|
Flotation tail of the I cleaning |
12.5 |
0.77 |
11.00 |
15.39 |
2.0 |
6.1 |
29.8 |
|
Flotation tail of the II recleaning |
16.9 |
0.46 |
29.04 |
2.35 |
1.7 |
21.9 |
6.1 |
|
Titanite concentrate (froth product of the II cleaning) |
37.6 |
0.40 |
37.80 |
0.39 |
3.7 |
63.3 |
2.3 |
|
Tailings |
7.8 |
0.96 |
2.08 |
25.31 |
1.6 |
0.7 |
30.6 |
|
Feed of titanite cycle |
83.8 |
0.54 |
25.34 |
7.60 |
9.8 |
94.6 |
98.7 |
|
Reagent regime 2:25 g/t CaCl2; rougher flotation 400 g/t СM1 + 120 g/t PABSA; |
|||||||
|
Froth product of control flotation |
4.5 |
0.34 |
29.03 |
2.80 |
0.3 |
5.9 |
1.9 |
|
Flotation tail of the I cleaning |
5.1 |
0.52 |
17.56 |
10.16 |
0.6 |
3.9 |
7.8 |
|
Flotation tail of the II recleaning |
14.6 |
0.53 |
30.56 |
0.93 |
1.7 |
19.9 |
2.0 |
|
Titanite concentrate (froth product of the II recleaning) |
34.3 |
0.89 |
34.30 |
0.30 |
6.6 |
52.6 |
1.6 |
|
Tailings |
25.8 |
0.36 |
8.72 |
21.60 |
2.0 |
10.0 |
84.2 |
|
Feed of titanite cycle |
84.3 |
0.61 |
24.53 |
7.66 |
11.2 |
92.2 |
97.5 |
The collecting HA mixture with addition of distilled tall oil (DTO) produced, as a result of two cleaning operations, a titanite concentrate with 37.8 % TiO2 content with 63.3 % feed ore recovery (Table 1). When using a mixture of tall oils as a collector (CM1: 70 % deciduous tall oil + 30 % pine tall oil) and PABSA, as a result of two cleaning operations, titanite concentrate was obtained with 34.3 % TiO2 content at recovery of 52.5 % of feed ore. It should be noted that in this case an attempt to improve the quality of the resulting concentrate by an additional cleaning operation leads to a significant decrease in product yield.
Mineral composition of beneficiation products obtained using reagent regime 1 is shown in Table 2. It is seen from Table 2 that the main titanium-bearing mineral of the Khibiny massif, titanite (39.3 % TiO2), is mainly concentrated in the titanite concentrate. The other minerals, practically free of titanium – nepheline, feldspars, and secondary minerals (cancrinite, sodalite, natrolite, fine-flake micas) as well as those containing titanium in smaller amounts – magnetite titanian (17.2 % TiO2), micas of the annite-phlogopite series (2-3 % TiO2), pyroxenes (1.3 % TiO2) [23], are concentrated in tailings.
Table 2
Mineral composition of beneficiation products during production of titanite concentrate
|
Mineral |
Content in products, wt. % |
Mineral |
Content in products, wt. % |
||
|
Titanite concentrate |
Tailings |
Titanite concentrate |
Tailings |
||
|
Titanite |
93.51 |
5.07 |
Sodalite |
0.25 |
1.37 |
|
Fluorapatite |
0.76 |
1.30 |
Natrolite |
0.31 |
3.72 |
|
Nepheline |
0.51 |
69.80 |
Ilmenite |
0.43 |
0.10 |
|
Pyroxenes |
0.96 |
1.43 |
Magnetite titanian |
0.06 |
0.40 |
|
Amphiboles |
0.67 |
1.65 |
Lamprophyllite |
0.21 |
0.47 |
|
Feldspars |
0.78 |
6.63 |
Micas |
1.34 |
4.46 |
|
Cancrinite |
0.20 |
3.59 |
Total |
100 |
100 |
Concentrate obtained by flotation method contains a significantly higher amount of titanium – 93.5 % titanite (37.8 % TiO2) compared to the concentrate obtained by acid leaching – 80 % titanite, 32 % TiO2. The content of other minerals in flotation concentrate is negligible, which allows using it without preliminary acid treatment [33] for further decomposition with sulfuric or hydrochloric acid to obtain precursors of various functional materials.
Conclusion
Mineralogical features of apatite-nepheline-titanite ores forming “titanite housing”
in the Khibiny massif, make it possible to recover high-quality titanite concentrate from them by flotation. Specific features of interaction of reagent with the hydroxamate group and titanite surface ensure the highest efficiency of flotation separation of titanite concentrate. The use of the most efficient flotation regime with a mixture of alkyl hydroxamic and carboxylic acids with addition of distilled tall oil ensures the production of titanite concentrate with titanite content of at least 93 % with extraction of 63 % TiO2 from the feed ore. The advantage of flotation separation of titanite concentrate in comparison with the chemical method based on sulfuric acid leaching is shown.
References
- Milyutin V.V., Nekrasova N.A., Yanicheva N.Yu. et al. Sorption of cesium and strontium radionuclides onto crystalline alkali metal titanosilicates. Radiochemistry. 2017. Vol. 59, p. 65-69. DOI: 201710.1134/s1066362217010088
- Thakkar J., Wissler B., Dudenas N. et al. Recovery of critical rare-earth elements using ETS-10 titanosilicate. Industrial & Engineering Chemistry Research. 2019. Vol. 58, p. 11121-11126. DOI: 10.1021/acs.iecr.9b02623
- Ortı'z-Oliveros H.B., Flores-Espinosa R.M., Ordon˜ez-Regil E., Ferna'ndez-Valverde S.M. Synthesis of a-Ti (HPO4)2-H2O and sorption of Eu(III). Chemical Engineering Journal. 2014. Vol. 236, p. 398-405. DOI: 10.1016/j.cej.2013.09.103
- Yve Xian Ooi, Kyaw Zay Ya, Keiichiro Maegawa et al. Incorporation of titanium pyrophosphate in polybenzimidazole membrane for medium temperature dry PEFC application. Solid State Ionics. 2020. Vol. 344, p. 115-140. DOI: 10.1016/j.ssi.2019.115140
- Vlaskin M.S., Grigorenko A.V., Shkolnikov E.I., Ilyukhin A.S. Gold-plated titanium vs carbon-impanted titanium as material for bipolar plates in PEM fuel cells. Surface Review and Letters. 2019. Vol. 26. N 8. N 1950038. DOI: 10.1142/S0218625X19500380
- Lin Peijian, Miao He, Wang Zhouhang et al. Research Progress on Titanium Based Perovskite Anodes for Solid Oxide Fuel Cell (SOFC). Materials Reports. Inorganic materials and ceramic matrix composites. 2020. Vol. 34. Iss. 5, p. 5032-5038 (in Chinese). DOI: 10.11896/cldb.19050165
- Shapovalov V., Guda A., Butova V. et al. Laboratory Operando XAS Study of Sodium Iron Titanite Cathode in the Li-Ion Half-Cell. Nanomaterials. 2021. Vol. 11. Iss. 1. N 156. DOI: 10.3390/nano11010156
- Martı'n-Yerga D., Carrasco-Rodrı'guez J., Fierro JLG et al. Copper-modified titanium phosphate nanoparticles as electrocatalyst for glucose detection. Electrochim Acta. 2017. Vol. 229, p. 102-111. DOI: 10.1016/j.electacta.2017.01.143
- Congqi Luan, Yong Zhou, Yongyi Liu et al. Effects of nano-SiO2, nano-CaCO3 and nano-TiO2 on properties and microstructure of the high content calcium silicate phase cement (HCSC). Construction and Building Materials. 2022. Vol. 314. Part A. N 125377. DOI: 10.1016/j.conbuildmat.2021.125377
- Chen J., Kou S.C., Poon C.S. Hydration and properties of nano-TiO2 blended cement composites. Cementand Concrete Composites. 2012. Vol. 34. Iss. 5, p. 642-649. DOI: 10.1016/j.cemconcomp.2012.02.009
- Decheng Feng, Ning Xie, Chunwei Gong et al. Portland Cement Paste Modified by TiO2 Nanoparticles: A Microstructure Perspective. Industrial & Engineering Chemistry Research. 2013. Vol. 52. Iss. 33, p. 11575-11582. DOI: 10.1021/ie4011595
- Smorokov A.A., Kantaev A.S., Bryankin D.V., Miklashevich A.A. Development of a low-temperature desiliconization method for the leucoxene concentrate of the Yarega deposit with a solution of ammonium hydrogen fluoride. ChemChemTech. 2022. Vol. 65. N 2, p. 127-133 (in Russian). DOI: 10.6060/ivkkt.20226502.6551
- Arkhipova Yu.A. Current state of titanium ore base of the Russian Far East and prospects for its development. Regionalnaya ekonomika: teoriya i praktika. 2010. Vol. 8. N 32, p. 36-43 (in Russian).
- Brylyakov Yu.Ye. Prospects for All-Round Utilization of Apatite-Nepheline Ores from the Khibini Deposits. Obogashchenie rud. 2005. N 3, p. 28-31 (in Russian).
- Ivanova V.A., Mitrofanova G.V. Aspects of comprehensive processing tehnology for stockpiled concentration wastes of apatite-nepheline ores. 15-th Balkan Mineral Processing Congress, 12-16 June, Sozopol, Bulgaria. St. Ivan Rilski, 2013. Vol. 2, p. 1112-1114.
- Mitrofanova G.V., Filimonova N.M., Andronov G.P., Rukhlenko E.D. Influence of Mineralogical-Technological Features of the Partomchorr Apatite-Containing Ores on the Choice of Reagent Flotation Regimes. Mining informational and analytical bulletin. 2017. N S23, p. 427-435 (in Russian). DOI: 10.25018/0236-1493-2017-10-23-427-435
- Gerasimova L.G., Shchukina E.S., Maslova M.V. et al. Preparation and Characteristics of Titanium Silicate Filler for Functional Materials. Inorganic Materials: Applied Research. 2020. Vol. 11, p. 903-907. DOI: 10.1134/S2075113320040103
- Gerasimova L.G., Maslova M.V., Shchukina E.S. Synthesis of Sorption Materials from Low Grade Titanium Raw Materials. Materials. 2022. Vol. 15. Iss. 5. N 1922. DOI: 10.3390/ma15051922
- Maslova M.V., Motov D.L., Gerasimova L.G. Production of a Complex Titano-Aluminium Material from Substandard Sphene Concentrate. ChemChemTech. 2006. Vol. 49. N 2, p. 63-66 (in Russian).
- Kalugin A.I., Pleshakov Yu.V., Gerasimova L.G., Nikolaev A.I. Innovative processing technologies for apatite-nepheline concentrates. Gornyi zhurnal. 2014. N 10, p. 69-72 (in Russian).
- Maslova M., Ivanenko V., Gerasimova L. et al. Synthesis of titanium phosphates from unconventional solid precursor and their ion-exchange and electrochemical properties. Journal of Materials Science. 2021. Vol. 56. Iss. 16, p. 9929-9950. DOI: 10.1007/s10853-021-05876-4
- Gerasimova L.G., Nikolaev A.I., Maslova M.V. et al. Titanite Ores of the Khibiny Apatite-Nepheline-Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis. Minerals. 2018. Vol. 8. Iss. 10. N 446. DOI: 10.3390/min8100446
- Borutskii B.E. Essays on fundamental and genetic mineralogy: 7. Evolution of notions on the genesis of the Khibiny apatite-nepheline deposits and the metasomatic hypothesis of their emplacement. Novye dannye o mineralakh. 2015. N 50, p. 129-167 (in Russian).
- Gerasimova L.G., Nikolaev A.I., Shchukina E.S., Maslova M.V. Titanite-Containing Mineral Compositions and Their Chemical Treatment with Preparation of Functional Materials. Materials. 2020. Vol. 13. Iss. 7. N 1599. DOI: 10.3390/ma13071599
- Samburov G.O., Shchukina E.S., Kiselev Yu.G. Titanium-Containing Concentrate from “Sphenite” Ore. Trudy Kolskogo nauchnogo tsentra RAN. 2017. Vol. 8. N 5-1, p. 148-154 (in Russian).
- Pleshakov Yu.V., Alekseev А.I., Brylyakov Yu.Ye., Nikolayev А.I. A Combined Processing Technology for Apatite-Nepheline Ores. Obogashchenie rud. 2004. N 2, p. 15-17 (in Russian).
- Gershenkop A.Sh., Gandrusov N.A., Andreeva A.I. Use of high molecular weight alkyl benzene sulfonates for flotation of nepheline. Tsvetnye metally. 1978. N 10, p. 110-112 (in Russian).
- Mitrofanova G.V., Chernousenko E.V., Kameneva Yu.S., Vishnyakova I.N. Testing of a Complexing Reagent on the Basis of Hydroxamic Acids by Floating Transition Metal Minerals. Vestnik Kolskogo nauchnogo tsentra RAN. 2019. Vol. 11. N 2, p. 95-104 (in Russian). DOI: 10.25702/KSC.2307-5228.2019.11.2.95-104
- Yaohui Yang, Longhua Xu, Yachuan Liu, Yuexin Han. Flotation separation of ilmenite from titanaugite using mixed collectors. Separation Science and Technology. 2016. Vol. 51. Iss. 11, p. 1840-1846. DOI: 10.1080/01496395.2016.1183678
- Xue X., Kanzaki M. Dissolution mechanisms of water in depolymerized silicate melts: Constraints from 1H and 29Si NMR spectroscopy and ab initio calculations. Geochimica et Cosmochimica Acta. 2004. Vol. 68. Iss. 24, p. 5027-5057. DOI: 10.1016/j.gca.2004.08.016
- Zhou F., Liu Q., Liu X., Li W. et al. Surface Electrical Behaviors of Apatite, Dolomite, Quartz, and Phosphate Ore. Frontiers in Materials. 2020. Vol. 7, p. 35. DOI: 10.3389/fmats.2020.00035
- Rappoport Z., Liebman J.F. The chemistry of hydroxylamines, oximes and hydroxamic acids. Wiley, 2008. Vol. 1, p. 1078.
- Brylyakov Yu.E., Bykov M.E., Skryabin A.N., Alekseev A.I. Hydrometallurgical technology for producing sphene and aegirine concentrates. Gornyi zhurnal. 2004. N 9, p. 66-68 (in Russian).
