Laboratory studies of transformation of porosity and permeability and chemical composition of terrigenous reservoir rocks at exposure to hydrogen (using the example of the Bobrikovskii formations in the oil field in the northeast Volga-Ural oil and gas province)
- 1 — Ph.D., Dr.Sci. Head of Laboratory Oil and Gas Research Institute ▪ Orcid
- 2 — Ph.D., Dr.Sci. Head of Department Perm National Research Polytechnic University ▪ Orcid
- 3 — Ph.D., Dr.Sci. Head of Laboratory Oil and Gas Research Institute ▪ Orcid
Abstract
The article describes the methodology for laboratory studies of reservoir rock exposure to hydrogen. The stages of sample research and the instruments used in the experiments are considered. A comparative analysis of the results of studies on porosity and permeability of core samples was performed. It was shown that after exposure to hydrogen, the porosity decreased by 4.6 %, and the permeability by 7.9 %. The analysis of correlation dependencies demonstrated a typical change in the relationship of these characteristics: after the samples exposure to hydrogen the scatter of the values increased and the correlation coefficient decreased, which indicates a change in the structure of the void space. Based on the research results, it was concluded that the decrease in porosity and permeability of the core samples occurred due to their minor compaction under the action of effective stresses. The chemical analysis of the rock showed no major difference in the composition of the basic oxides before and after exposure to hydrogen, which points to the chemical resistance of the studied formation to hydrogen. The experimental results showed that the horizon under consideration can be a storage of the hydrogen-methane mixture.
Funding
The article was prepared as part of the government ordered theme “Scientific substantiation of the influence of hydrochemical and microbiological processes on the development of corrosion phenomena in case of hydrogen and methane co-occurrence in a wide range of concentrations in geological bodies of various types” (FMME-2022-0007, N 122022800276-2).
Introduction
Lately, increasing attention was paid to environmentally friendly production technologies and reducing carbon dioxide emissions into the atmosphere including the alternative energy sources and replacing hydrocarbons with other energy products. Thus, in publication [1], the authors note the relevance of the global warming problem and consider the technologies for capturing and disposal of carbon dioxide. It was noted that in Russia such technologies should be introduced into production by the governmental programmes and should be based on foreign experience.
Hydrogen is expected to be used as one of the alternative fuels. In this case, problems related to its production and transportation arise. Articles [2-4] describe numerous methods for producing this type of fuel which are characterized by different energy efficiency and environmental friendliness. The authors of these works recommend using the technologies that will not involve carbon dioxide emission. The researchers in publications [5-7] point out that the transportation of hydrogen requires converting it to a higher density (liquefaction) as well as increasing the safety of tanks and transport systems.
A special aspect of hydrogen energy is the choice of gas storage facilities. As was shown in scientific articles [8-10], possible formations for the storage of the hydrogen-methane mixture are salt caverns. However, such reservoirs have both advantages, and disadvantages. An advantage is a good tightness, and the main disadvantages are labour-consuming creation, cost and small volumes of stored fuel, since large salt caverns collapse under external loads.
Some authors consider the use of the existing underground gas storage facilities (UGS), in which natural gas is traditionally stored [11, 12]. For large gas volumes it is recommended to use the depleted gas fields, aquifers, or storage facilities currently used to store methane.
A major problem associated with hydrogen storage is the embrittlement of steel well strings and downhole equipment. Articles [13-15] provide evidence that under the influence of hydrogen, physicochemical processes occur in traditionally applied steel grades leading to cracking and then to deterioration of their stress-strain properties. The consequences of such effects are unforeseen emergencies in wells and with the equipment used.
Vital activity of bacteria absorbing hydrogen also has a negative effect. The experts [16-18] noted that some bacterial species found in the reservoir are capable of converting hydrogen into hydrogen sulphide which is an extremely aggressive gas, and its impact on the well structure and equipment can lead to destruction.
When hydrogen is stored in reservoir beds, it can enter into chemical interaction with minerals of the rock matrix and the overlying cap rock. Thus, the authors of the articles [19-21] provide data showing that hydrogen reacts with pyrite, minerals containing aluminium as well as with carbon dioxide and sulphates dissolved in water, resulting in the production of methane and hydrogen sulphide. The consequence of such effects can be the transformation of porosity and permeability (reservoir properties) of the formation. Publications [22-24] show that under the action of hydrogen, a twofold change in the porosity of core samples can occur. Such effects depend on the lithological composition of rocks, and, therefore, it is recommended to store gas primarily in terrigenous reservoirs without admixtures of clays and carbonates.
Similar to most of traditional underground gas storages with methane, in case of storing hydrogen, a complex geodynamic situation can occur in the area of the operating facilities. As shown in articles [25-27], changes in formation pressures during gas injection and pumping-out cycles can lead to deformations of the earth’s surface, and, therefore, in such UGS it is necessary to arrange geodynamic polygons and monitor the rock mass movements in the area of the storage.
The analysis of publications showed that the influence of hydrogen on changes in porosity and permeability of reservoir rocks as well as on the transformation of the chemical composition of the rock matrix is so far inadequately studied. Therefore, one of the important and inadequately studied aspects of this problem was considered related to the influence of hydrogen on the reservoir properties and changes in the chemical composition of rocks of the investigated formation. The studies were accomplished using the example of terrigenous deposits of the Bobrikovskii horizon in the Volga-Ural oil and gas province. Gas in the Karashurskoe UGS in the Republic of Udmurtia is stored in such formation [28-30]. The methodology for studying core samples was developed, and the unit was manufactured for a long-term exposure of samples to hydrogen. The results of lithological and petrographic studies of thin sections of core samples are presented. To identify the effects of hydrogen on the transformation of natural properties of the reservoir rock, a comparative analysis of the results of laboratory studies of porosity, permeability of samples and the chemical composition of the rock matrix was conducted.
Methodology
The impact of hydrogen injection on natural properties of reservoir was studied using the example of the Bobrikovskii terrigenous formations – a geological body of underground gas storage in the Volga-Ural oil and gas province. Since it was not possible to use the core from the Karashurskoe UGS, the core from a similar Bobrikovskii formation in one of the oil fields in the northeast of the Volga-Ural oil and gas province was taken for laboratory experiments (Fig.1).

Fig.1. Source core material and samples after drilling out
The core was taken from the intervals with the highest porosity and permeability as well as from the depth closest to the occurrence depth of the production zone in the Karashurskoe UGS. Thus, the core was taken from the depth of 1,488.4-1,489.8 m. Twenty-four core columns were drilled from the source core material (Fig.1), half of which had standard dimensions (length and diameter 3 cm), and the other half had non-standard length (6 cm) and diameter 3 cm. Samples with a non-standard length were further used to study the stress-strain properties of rocks by the static method before and after exposure to hydrogen.
Samples with obvious cracks were rejected and not used in further studies. Thus, the total number of samples was reduced to 20. After drilling out the samples, their standard preparation was accomplished: extraction with an alcohol-benzene mixture for 20 days using Soxhlet apparatus and drying.
Figure 1 shows the appearance of the prepared core samples which were divided into five groups of four samples (Table 1). Each group consisted of two standard samples and two “long” samples. It is implied that for two standard samples and two “long” samples from each group, the results of determining the reservoir properties (porosity and permeability) will be compared, and further also the stress-strain properties (elastic modulus, Poisson’s ratio, tensile and compressive strength) before and after exposure to hydrogen.
Table 1
Geometric characteristics of core samples and their porosity and permeability determined before and after exposure to hydrogen
|
Group number |
Sample number |
Length h, cm |
Diameter d, cm |
Before exposure to hydrogen |
After exposure to hydrogen |
Absolute change |
Relative change |
||||
|
Kp, % |
Kper, mD |
Kp, % |
Kper, mD |
ΔKp, % |
ΔKper, mD |
ΔKp, % |
ΔKper,% |
||||
|
1 |
1 |
6.03 |
3.00 |
22.4 |
720 |
|
|
|
|
|
|
|
1 |
19 |
5.89 |
3.00 |
23.1 |
848 |
21.3 |
757 |
–1.8 |
–91 |
–7.79 |
–10.73 |
|
1 |
11/2 |
3.03 |
3.02 |
23.3 |
759 |
|
|
|
|
|
|
|
1 |
2/1 |
3.01 |
3.00 |
22.4 |
739 |
22.3 |
692 |
–0.1 |
–47 |
–0.45 |
–6.36 |
|
2 |
4 |
5.89 |
2.99 |
22.4 |
664 |
|
|
|
|
|
|
|
2 |
12 |
5.84 |
3.00 |
22.2 |
706 |
21.5 |
646 |
–0.7 |
–60 |
–3.15 |
–8.50 |
|
2 |
2/2 |
2.90 |
3.00 |
22.9 |
686 |
|
|
|
|
|
|
|
2 |
14 |
2.96 |
2.99 |
22.9 |
686 |
22.5 |
644 |
–0.4 |
–42 |
–1.75 |
–6.12 |
|
3 |
6 |
6.03 |
3.01 |
21.3 |
604 |
|
|
|
|
|
|
|
3 |
15 |
6.01 |
2.99 |
21.4 |
518 |
20.7 |
495 |
–0.7 |
–23 |
–3.27 |
–4.44 |
|
3 |
13 |
2.98 |
2.99 |
23.0 |
643 |
|
|
|
|
|
|
|
3 |
5/1 |
2.87 |
2.99 |
22.8 |
644 |
21.1 |
603 |
–1.7 |
–41 |
–7.46 |
–6.37 |
|
4 |
7 |
5.97 |
2.99 |
22.1 |
612 |
|
|
|
|
|
|
|
4 |
3 |
6.02 |
3.00 |
22.6 |
634 |
22.0 |
618 |
–0.6 |
–16 |
–2.65 |
–2.52 |
|
4 |
11/1 |
3.00 |
3.02 |
22.7 |
717 |
|
|
|
|
|
|
|
4 |
8/1 |
2.98 |
3.00 |
22.4 |
669 |
19.4 |
603 |
–3.0 |
–66 |
–13.39 |
–9.87 |
|
5 |
16 |
5.9 |
2.99 |
22.3 |
496 |
|
|
|
|
|
|
|
5 |
21 |
5.89 |
3.02 |
21.6 |
463 |
21.0 |
448 |
–0.6 |
–15 |
–2.78 |
–3.24 |
|
5 |
2/5 |
2.93 |
3.00 |
22.5 |
611 |
|
|
|
|
|
|
|
5 |
8/2 |
2.91 |
3.01 |
22.3 |
655 |
21.7 |
625 |
–0.6 |
–30 |
–2.69 |
–4.58 |
|
Average value |
22.4 |
656 |
21.3 |
612 |
–1.07 |
–44.6 |
–4.74 |
–6.46 |
|||
Table 2 shows a detailed programme of sample studies. Research stages:
Stage 1. After standard preparation of samples, their porosity and gas permeability were determined in the conditions close to atmospheric ones: open porosity Kp and absolute permeability Kper. Studies of reservoir properties were accomplished after GOST 26450.2-85 using a PIK-PP unit (AO “Geologiya”). For a reliable determination of these properties, the samples were subjected to the minimum overburden pressure of 2.5 MPa to prevent gas flow around the side surface of the samples. The studies were performed to identify the scatter in the reservoir properties of core samples and determine the representativeness of their selection.
Table 2
Main stages of the research programme, equipment and determined parameters for each group of four samples
|
Stage |
Content of research stage |
Unit used |
Determined parameters |
|
1 |
Samples are extracted and dried, open porosity and absolute gas permeability are determined at effective stresses close to atmospheric conditions (overburden pressure 2.5 MPa)
|
PIK-PP |
Kper, Kp |
|
2 |
Lithological and petrophysical studies of thin sections of core samples
|
Polarising microscope Leica DM 2700P |
Lithological and petrophysical properties |
|
3 |
For three crushed samples, the chemical analysis of rock is performed, the composition of the main rock-forming oxides: Fe2O3, MnO, TiO2, Al2O3, SiO2, CaO, MgO, Na2O, K2O, P2O5, Stot |
Spectroscan MAKS-GV |
Mass fractions of oxides |
|
4 |
From each group, one “long” sample, one standard sample and part of the crushed core are placed in a cylinder, into which hydrogen is then injected. Samples are kept in the cylinder for 7 days
|
Cylinder for injecting hydrogen and keeping samples |
|
|
5 |
For samples removed from the cylinder, the porosity and gas permeability are determined at effective stresses close to atmospheric conditions (overburden pressure 2.5 MPa)
|
PIK-PP |
Kper, Kp |
|
6 |
For samples extracted from the cylinder, chemical analysis of the rock is performed, composition of the main rock-forming oxides: Fe2O3, MnO, TiO2, Al2O3, SiO2, CaO, MgO, Na2O, K2O, P2O5, Stot |
Spectroscan MAKS-GV |
Mass fractions of oxides |
Stage 2. Determination of minerals making up the rock under study.
Stage 3. Studies were conducted before exposure to hydrogen, and some of the samples were crushed and thoroughly mixed for subsequent analysis. A total of three crushed core samples were studied, since it was assumed that due to crushing and thorough mixing, their composition should be similar. For them, the chemical analysis of basic oxides was accomplished without the influence of hydrogen after GOST 5382-2019, clause 7 (weight loss on ignition – LOI), clause 23 (X-ray spectral method for determining the elements) using X-ray fluorescence spectrometer MAKS-GV.
Stage 4. Long-term exposure of samples to hydrogen using a unit consisting of a cylinder with hydrogen, a pressure reducer with pressure sensors, and a sealed cylinder (Fig.2).
To expose core samples to hydrogen, gas was supplied from the cylinder with compressed hydrogen with a capacity of 40 dm3 and the volume of gas in the cylinder was 6.3 m3 (Fig.2). Gas in the cylinder had the following characteristics:
- volume fraction of hydrogen in terms of dry gas – not less than 99.99%;
- total volume fraction of oxygen and nitrogen – not more than 0.01%;
- mass concentration of water vapour at a temperature of 20 °C and a pressure of 101.3 kPa (760 mm Hg) in the cylinder under pressure – not more than 0.2 g/m3;
- pressure in the cylinder at a temperature of 20 °C – 14.7±0.5 MPa.
Since hydrogen in the cylinder was under high pressure, for reducing it, a reducer was connected to the cylinder with two sensors displaying gas pressure at the inlet and outlet of the reducer. The approximate gas pressure after its reduction was 0.6-0.7 MPa.
For interacting with hydrogen, core samples were placed in a specially prepared cylinder (Fig.2) with inlet and outlet openings for gas supply and removal. For each group studied, one standard sample and one “long” sample were placed in the cylinder as well as a sample of crushed rock moistened with distilled water. To prevent the crushed core from being carried away along with gas, it was placed in a special sieve.
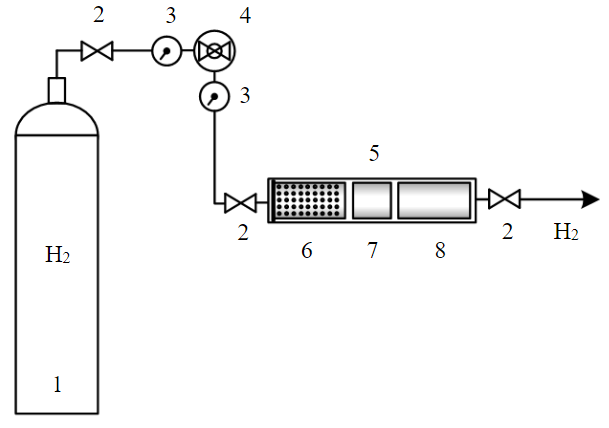
Fig.2. Scheme of the unit for holding core samples exposed to hydrogen 1 – balloon with hydrogen; 2 – valves; 3 – pressure sensors; 4 – pressure reducer with valve; 5 – cylinder with core samples; 6 – sieve with crushed rock; 7 – standard core sample (diameter, length 3 cm); 8 – “long” core sample (diameter 3 cm, length 6 cm)
The reducer and the cylinder were connected to the cylinder with core samples after a certain volume of hydrogen had passed through the cylinder to displace the air. Then, both valves of the cylinder were closed, and the rock was exposed to hydrogen for seven days, while gas in the cylinder was renewed every 24 hours. In total, five groups of samples were exposed to hydrogen, i.e., five standard samples, five “long” and five crushed rock samples to study changes in the chemical composition.
Stages 5, 6. After exposing the samples to hydrogen, the porosity and gas permeability were again determined, and the chemical analysis of basic oxides was performed.
Results
The results of determining open porosity and absolute permeability of the samples (stage 1) as well as their geometric characteristics are given in Table 1. Porosity of the samples ranged within 21.3-23.3 %, and permeability from 463 to 848 mD and averaged 22.4 % and 653.7 mD, respectively.
Correlation dependence of absolute gas permeability of core samples on open porosity is shown in Fig.3 (blue circles). This graph shows that there is a fairly close relationship between these cha-racteristics and correlation coefficient of 0.68, which points to the homogeneity of the samples selected for research.
Figure 4 shows the photos of some studied thin sections of core samples (stage 2). The study of thin sections gave the following main results:
- The samples consist predominantly of fine-grained silty sandstones with a quartz and feldspathic-quartz mineral composition. Rock structure is fine-grained silty-psammitic with grain sizes of 0.05-0.2 mm dominated by 10-30% of 0.1-0.16 mm grains of silty fraction. Grains are irregular, subisometric, elongated, semi-rounded. Texture is microlayered due to orientation of some elongated fragments.
- The rock is represented by quartz grains (82-95%), feldspar grains (to 5 %), mica (to 8 %). Quartz is characterized by an irregular elongated shape, sometimes with traces of regeneration (0.003-0.015 mm). Sometimes, grains are dissolved along the periphery, that is why they acquire uneven edges. Feldspars are represented by plagioclase with weak pelitization, partially dissolved. As for micas, blades of muscovite occur, and chlorite flakes are also found.
- The rock is dominated by indentation cementation – a cementless contact connection of quartz grains and fragments which is characterized by a conformal structure. Accessory minerals are pyroxene grains 0.08-0.18 mm in size.
- Authigenic minerals – single hydromica flakes less than 0.05 mm in size and calcite crystals 0.25-0.3 mm in size (less than 1%). Post-sedimentation transformations – indentation structure and formation of conformal structures due to quartz regeneration and compaction of fragments.
- The void space of rock (~3-5%) is unevenly represented by intergranular isolated hollow pores, presumably of secondary origin, of irregular shape, 0.06-0.25 mm in size.
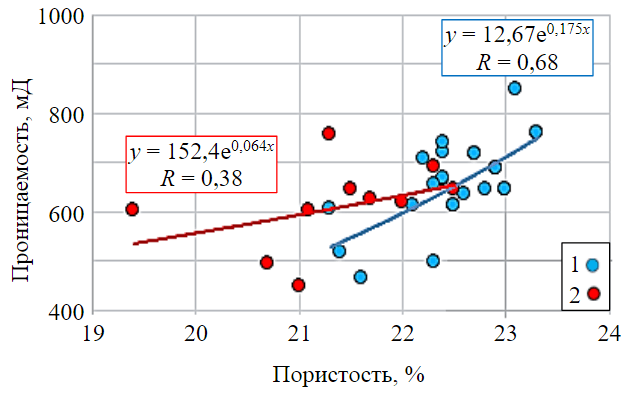
Fig.3. Dependence of permeability on porosity of core samples before (1) and after (2) exposure to hydrogen
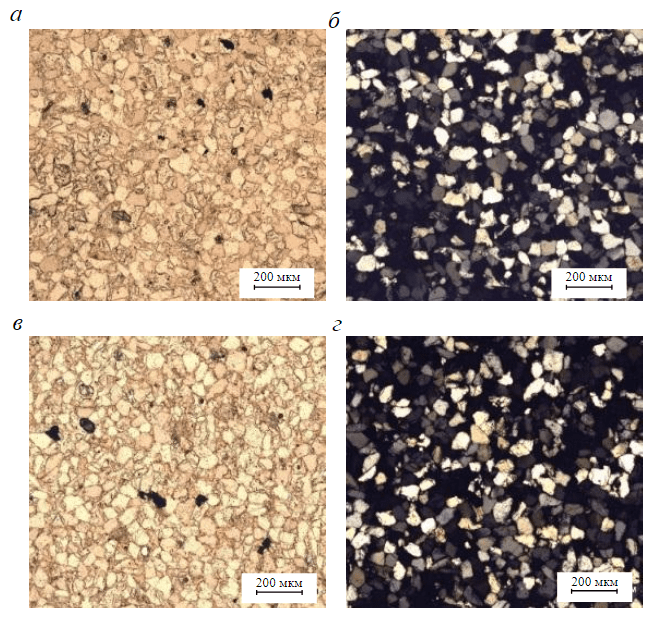
Fig.4. Photos of the investigated thin sections of core samples N 1 (a, b) and N 4 (c, d): a, c – without analyzer; b, d – with analyzer
Articles [21, 31, 32] show that quartz particles for the mining and geological conditions of the UGS practically do not interact with hydrogen.
The results of chemical analysis of the rock (stage 3) are presented in Table 3 and Fig.5. As can be seen from these data, the samples have the chemical composition characteristic of a terrigenous reservoir.
Figure 5 shows the averaged composition of oxides for three samples of crushed rock, while the composition with and without silicon oxide is plotted separately, since its content is much higher than that of other oxides. The chemical composition of the investigated samples is dominated by silicon oxide, the amount of which is on average 96.64 %, the amount of other oxides, together with weight loss on ignition, is 2.01 % (Fig.5, a).
Table 3
Results of determining the chemical composition of rocks before and after exposure to hydrogen
|
Sample number |
Content in rock, % |
|||||||||||
|
Mass fraction of a chemical element in terms of oxide |
LOI |
|||||||||||
|
Fe – Fe2O3 tot |
Mn – MnO (II) |
Ti – TiO2 |
Al – Al2O3 |
Si – SiO2 |
Ca – CaO |
Mg – MgO |
Na – Na2O |
K – K2О |
P – P2O5 |
S – Stot |
||
|
|
Before exposure to hydrogen |
|||||||||||
|
1 |
0.81 |
< 0.01 |
0.28 |
< 0.1 |
96.44 |
0.32 |
0.13 |
0.20 |
0.22 |
0.02 |
0.23 |
1.08 |
|
2 |
0.77 |
< 0.01 |
0.28 |
< 0.1 |
96.83 |
0.29 |
0.03 |
0.22 |
0.21 |
0.02 |
0.22 |
1.09 |
|
3 |
0.75 |
< 0.01 |
0.24 |
< 0.1 |
96.65 |
0.25 |
0.02 |
0.06 |
0.20 |
0.02 |
0.25 |
1.10 |
|
Average value |
0.78 |
|
0.27 |
|
96.64 |
0.29 |
0.06 |
0.16 |
0.21 |
0.02 |
0.23 |
1.09 |
|
|
After exposure to hydrogen |
|||||||||||
|
1 |
0.98 |
0.01 |
0.28 |
< 0.1 |
95.01 |
0.46 |
0.11 |
0.14 |
0.25 |
0.02 |
0.29 |
2.58 |
|
2 |
0.72 |
< 0.01 |
0.24 |
< 0.1 |
95.73 |
0.25 |
0.16 |
0.16 |
0.14 |
0.02 |
0.21 |
1.18 |
|
3 |
0.75 |
0.01 |
0.23 |
< 0.1 |
97.62 |
0.31 |
0.10 |
0.11 |
0.14 |
0.02 |
0.18 |
1.26 |
|
4 |
0.82 |
< 0.01 |
0.25 |
< 0.1 |
95.64 |
0.24 |
< 0.1 |
0.22 |
0.21 |
0.02 |
0.32 |
1.23 |
|
5 |
0.72 |
< 0.01 |
0.21 |
< 0.1 |
97.11 |
0.28 |
< 0.1 |
0.25 |
0.26 |
0.02 |
0.44 |
1.13 |
|
Average value |
0.80 |
0.01 |
0.24 |
|
96.22 |
0.31 |
0.12 |
0.18 |
0.20 |
0.02 |
0.29 |
1.48 |
|
Absolute change |
0.02 |
|
–0.02 |
|
–0.42 |
0.02 |
0.06 |
0.01 |
–0.01 |
0.00 |
0.06 |
0.39 |
As can be seen from Fig.5, b, iron oxide is ranking second after silicon oxide in quantitative composition (0.78 %). The samples also contained (in order of decreasing content): calcium oxide (0.29 %), titanium oxide (0.27 %), sulphur oxides (0.23 %), potassium oxide (0.21 %), sodium oxide (0.16 %), magnesium oxide (0.06 %), and phosphorus oxide (0.02 %).
After a long-term exposure of the samples to hydrogen (stages 4, 5), it is possible to compare changes in reservoir properties before and after the exposure (see Table 1, Fig.3 (red dots), Fig.6). For the convenience of comparison, each graph (Fig.6) shows a dotted line of equal values. Comparing the values obtained experimentally, with this straight line it is possible to immediately determine in which direction – decrease or increase – a change in a certain characteristic occurred after the exposure to hydrogen. Thus, if the dots are below the line of equal values, there was a decrease in the considered rock property; if the dots are above the line, then, on the contrary, there was an increase in this property. Also, for convenience, Fig.6 shows an approximating function of a linear form starting from the origin of coordinates. Based on the coefficient at variable x of the given function, it is possible to determine how much this characteristic changes.
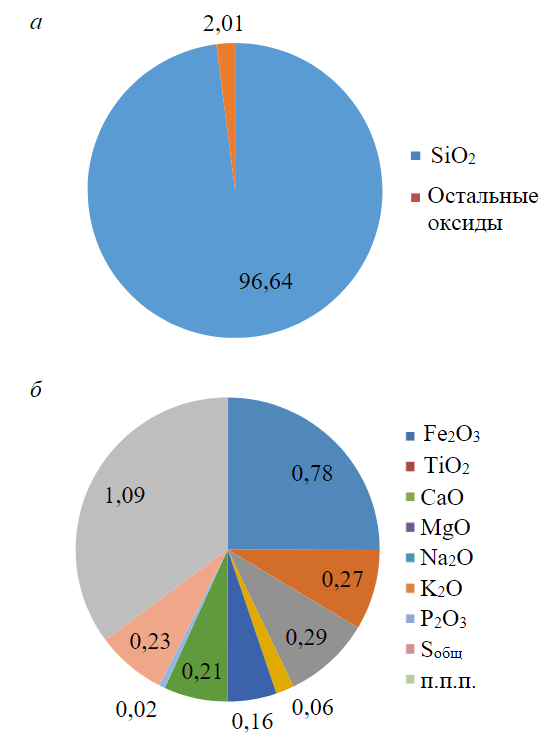
Fig.5. Results of determining the chemical composition of samples before exposure to hydrogen: a – all oxides, except silicon, are combined into one value; b – silicon oxide is excluded
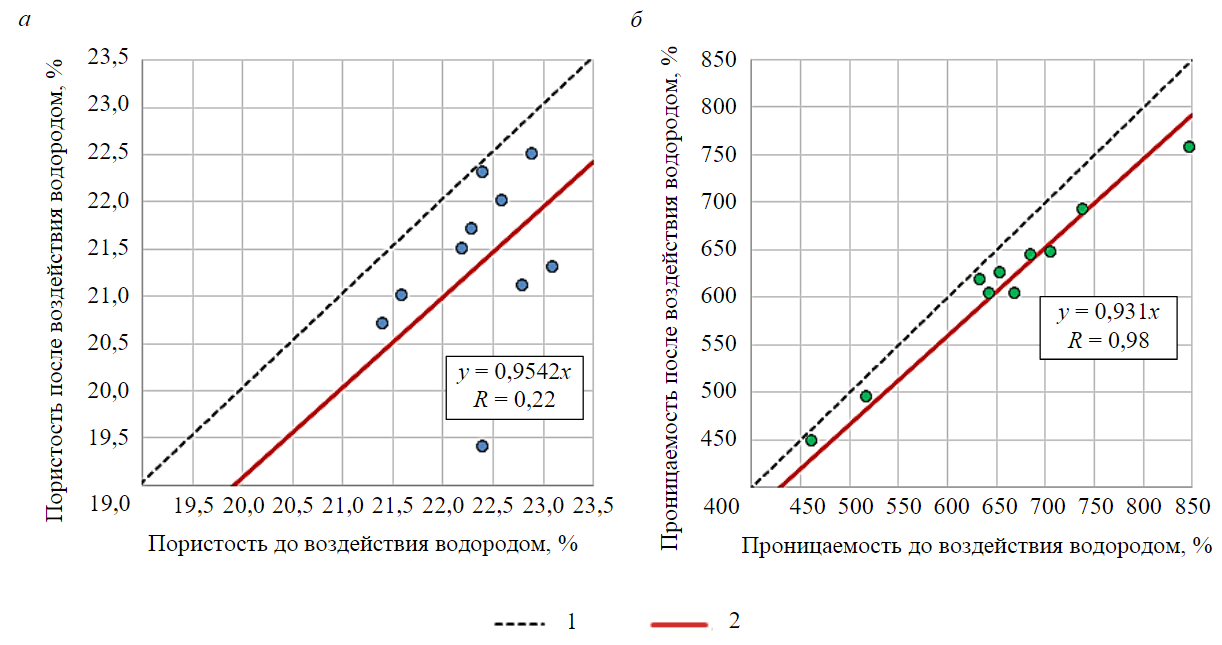
Fig.6. Comparison of porosity (a) and permeability (b) of core samples before and after exposure to hydrogen 1 – line of equal values; 2 – approximation
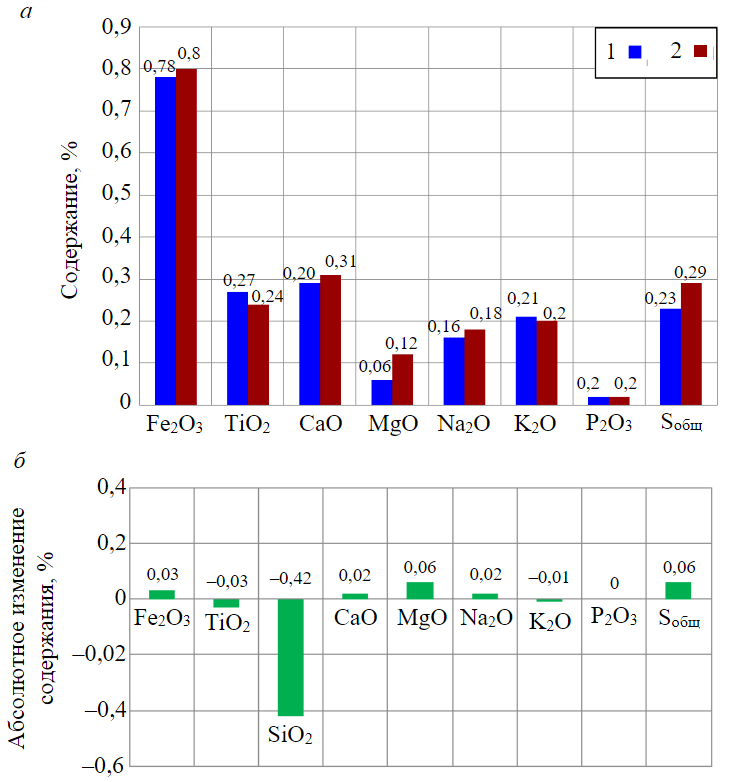
Fig.7. Average composition of basic oxides (silicon oxide excluded) before and after exposure to hydrogen (a) and an absolute change in their contents after exposure to hydrogen (b) in the total mass of the sample 1 – before exposure; 2 – after exposure
Table 3 presents the results of studying the che-mical composition of rock before and after exposure to hydrogen (stage 6). For a more convenient data analysis, Fig.7 shows the composition of basic oxides in percentage terms as well as their absolute change.
Discussion of results
From the comparison of the results of determining the porosity and permeability of core samples before and after exposure to hydrogen (see Fig.5), it follows that after exposure to gas, the porosity and permeability decrease by 4.6 and 7.9 %, respectively. Thus, it can be concluded that after the exposure to hydrogen the samples are compacted. According to the authors of the work, such compaction was a consequence of weakening of rocks under the action of hydrogen. At the same time, when measuring the porosity and permeability of samples after such exposure and creating the overburden pressure of 2.5 MPa there occurred an additional compaction of core samples despite the fact that they had already been subjected to the impact of compressive load during the first cycle of measurements of these properties before exposure to hydrogen. Presumably, such exposure disturb ed the strength of intergranular contacts, which led to rock matrix weakening. The relationship between changes in both porosity and permeability after exposure to hydrogen is quite natural, since when the volume of the void space changes, the rock compressibility also changes [33-35], which leads to variations in its porosity and permeability under changes in effective stresses.
In addition to the correlation dependencies shown in Fig.6, it was found that the dependence of permeability on porosity changed significantly after exposure to hydrogen (see Fig.3): the correlation coefficient decreased, and the scatter of permeability values became larger, and with decreasing porosity, the permeability did not decrease significantly.
In conclusion, it is necessary to consider the results of comparing the chemical analyses of crushed rock samples before and after exposure to hydrogen (Table 3, Fig.7). Figure 7 shows the average values of the results of determining basic oxides for three samples before exposure to hydrogen and five samples after exposure to hydrogen. The change in the chemical composition of rocks before and after exposure to hydrogen is negligible and tends both to increase and decrease. The exception is silicon oxide, but its content in the rock is maximum. In general, such changes can be caused both by changes in the composition of samples and by measurement errors.
Thus, based on the comparison of the results of chemical analysis of rock composition, it can be concluded that the effect of hydrogen on the chemical composition of core samples is minor. This result suggests that the geological body under study (Bobrikovskii horizon) can be the formation for the storage of the hydrogen-methane mixture. At the same time, it is possible to additionally confirm this conclusion only by conducting more detailed studies with a larger number of samples and a longer time of exposure to hydrogen (to one month or more).
Conclusion
As part of the work, the methodology and results of laboratory studies of hydrogen exposure influence on the transformation of reservoir properties and chemical composition of terrigenous reservoir rocks are considered using the example of the Bobrikovskii horizon in the Volga-Ural oil and gas bearing area. The research results allow drawing the following main conclusions:
- The authors of the article produced an experimental unit and developed a special programme for studying core samples, which makes it possible to investigate the reservoir properties, density, dynamic characteristics, stress-strain properties, and chemical composition of core samples before and after exposure to hydrogen. The research methodology was tested on terrigenous core samples taken from the Bobrikovskii formation in one of the oil fields in the Volga-Ural province.
- Lithological and petrophysical analysis showed that the investigated core samples were mainly composed of quartz grains; there was also a minor amount of feldspar and muscovite in the rock.
- During the experiments, the reservoir properties of the core samples were studied before and after exposure to hydrogen. The research results showed that after exposure to hydrogen, the porosity and permeability decreased by 4.6 and 7.9%, respectively.
- According to the authors of the article, the decrease in the porosity and permeability of samples was due to weakening of rocks under the action of hydrogen. At the same time, when measuring the porosity and permeability of the samples after exposure to hydrogen and generation of the overburden pressure of 2.5 MPa, an additional compaction of core samples occurred despite the fact that they had already been subjected to such an impact during the first cycle of measurements of these properties before exposure to hydrogen. Exposure to hydrogen disturbed the strength of intergranular contacts, which led to weakening of the rock. At the same time, it should be noted that the above values of a decrease in porosity and permeability were not so significant and should not have a major effect on the process of gas injection and removal, given that hydrogen is much more mobile than natural gas.
- The results of comparing data from chemical analysis of the content of basic oxides showed that the change in the chemical composition of rocks before and after exposure to hydrogen was negligible and could be caused by changes in the composition of samples and measurement error. Based on these results, it follows that the investigated formation is chemically resistant to hydrogen. This is supported by the fact that the samples contain a large amount (96.64%) of silicon oxide, which, in the conditions of the formation under study does not interact with hydrogen.
- Analysis of the results of studying porosity and permeability of core samples and the chemical composition of rocks shows that the influence of hydrogen on the considered reservoir rock is insignificant. The studied formation (Bobrikovskii horizon in the Volga-Ural oil and gas province) can be used for the storage of the hydrogen-methane mixture. However, this conclusion can only be confirmed by conducting more detailed studies with a large number of samples and a longer time of exposure to hydrogen.
References
- Fedoseev S.V., Tcvetkov P.S. Key Factors of Public Perception of Carbon Dioxide Capture and Storage Projects. Journal of Mining Institute. 2019. Vol. 237, p. 361-368. DOI: 10.31897/PMI.2019.3.361
- Litvinenko V.S., Tsvetkov P.S., Dvoynikov M.V., Buslaev G.V. Barriers to implementation of hydrogen initiatives in the context of global energy sustainable development. Journal of Mining Institute. 2020. Vol. 244, p. 428-438. DOI: 10.31897/PMI.2020.4.5
- Agyekum E.B., Nutakor C., Agwa A.M., Kamel S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affec-ting Their Scale-Up and Its Role in Future Energy Generation. Membranes. 2022. Vol. 12. Iss. 2. N 173. DOI: 10.3390/membranes12020173
- Tarhan C., Çil M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. Journal of Energy Storage. 2021. Vol. 40. N 102676. DOI: 10.1016/j.est.2021.102676
- Liuxi Cai, Guangqian Bai, Xiufeng Gao et al. Experimental investigation on the hydrogen embrittlement characteristics and mechanism of natural gas-hydrogen transportation pipeline steels. Materials Research Express. 2022. Vol. 9. N 4. N 046512. DOI: 10.1088/2053-1591/ac6654
- Hafsi Z., Mishra M., Elaoud S. Hydrogen embrittlement of steel pipelines during transients. Procedia Structural Integrity. 2018. Vol. 13, p. 210-217. DOI: 10.1016/j.prostr.2018.12.035
- Khare A., Vishwakarma M., Parashar V. A Review on Failures of Industrial Components due to Hydrogen Embrittlement & Techniques for Damage Prevention. International Journal of Applied Engineering Research. 2017. Vol. 12. N 8, p. 1784-1792.
- Popov S.N., Chernyshov S.E. Coupled mechanical and chemical and geodynamic problems arising during the operation of underground gas storage facilities with a mixture of hydrogen and methane. Actual Problems of Oil and Gas. 2020. Iss. 3 (30), p. 32-43. DOI: 10.29222/ipng.2078-5712.2020-30.art4
- Jinlong Li, Xilin Shi, Chunhe Yang et al. Repair of irregularly shaped salt cavern gas storage by re-leaching under gas blanket. Journal of Natural Gas Science and Engineering. 2017. Vol. 45, p. 848-859. DOI: 10.1016/j.jngse.2017.07.004
- Pellet F.L. Rock mechanics and environmental engineering for energy and geo-resources. EUROROCK 2018 International European Rock Mechanics Symposium, 22-26 May 2018, Saint Petersburg, Russia. Geomechanics and Geodynamics of Rock Masses, 2018. Vol. 1, p. 87-93.
- Gasanzade F., Bauer S., Pfeiffer W.T. Sensitivity analysis of gas leakage through a fault zone during subsurface gas storage in porous formations. European Geosciences Union General Assembly 2019, 7-12 April 2019, Vienna, Austria. Advances in Geosciences. 2019. Vol. 49, p. 155-164. DOI: 10.5194/adgeo-49-155-2019
- Pfeiffer W.T., Al Hagrey S.A., Köhn D. et al. Porous media hydrogen storage at a synthetic, heterogeneous field site: numerical simulation of storage operation and geophysical monitoring. Environmental Earth Sciences. 2016. Vol. 75. Iss. 16. N 1177. DOI: 10.1007/s12665-016-5958-x
- Martin M.L., Connolly M.J., DelRio F.W., Slifka A.J. Hydrogen embrittlement in ferritic steels. Applied Physics Reviews. 2020. Vol. 7. N 4. N 041301. DOI: 10.1063/5.0012851
- Shadravan A., Amani M. Impacts of Hydrogen Embrittlement on Oil and Gas Wells: Theories behind Premature Failures. SPE Gas & Oil Technology Showcase and Conference, 21-23 October 2019, Dubai, UAE. OnePetro, 2019. N SPE-198588-MS. DOI: 10.2118/198588-MS
- Zvirko O., Tsyrulnyk O., Lipiec S., Dzioba I. Evaluation of Corrosion, Mechanical Properties and Hydrogen Embrittlement of Casing Pipe Steels with Different Microstructure. Materials. 2021. Vol. 14. Iss. 24. N 7860. DOI: 10.3390/ma14247860
- Nazina T.N., Abukova L.A., Tourova T.P. et al. Diversity and Possible Activity of Microorganisms in Underground Gas Storage Aquifers. Microbiology. 2021. Vol. 90. Iss. 5, p. 589-600. DOI: 10.31857/S002636562105013X
- Dopffel N., Jansen S., Gerritse J. Microbial side effects of underground hydrogen storage – Knowledge gaps, risks and opportunities for successful implementation. International Journal of Hydrogen Energy. 2021. Vol. 46. Iss. 12, p. 8594-8606. DOI: 10.1016/j.ijhydene.2020.12.058
- Gregory S.P., Barnett M.J., Field L.P., Milodowski A.E. Subsurface Microbial Hydrogen Cycling: Natural Occurrence and Implications for Industry. Microorganisms. 2019. Vol. 7. Iss. 2. N 53. DOI: 10.3390/microorganisms7020053
- Abramova O.P., Filippova D.S. Geobiological Features of Storage Hydrogen-Methane Mixtures in Underground Reservoirs. SOCAR proceedings. 2021. SI2, p. 66-74. DOI: 10.5510/OGP2021SI200548
- Abukova L.A., Abramova O.P. Prediction of hydrogeochemical effects in clayey cap rock during underground storage of hydrogen with methane. Georesursy. 2021. Vol. 23. N 1, p. 118-126. DOI: 10.18599/grs.2021.1.13
- Yekta A.E., Pichavant M., Audigane P. Evaluation of geochemical reactivity of hydrogen in sandstone: Application to geological storage. Applied geochemistry. 2018. Vol. 95, p. 182-194. DOI: 10.1016/j.apgeochem.2018.05.021
- Flesch S., Pudlo D., Albrecht et al. Hydrogen underground storage – Petrographic and petrophysical variations in reservoir sandstones from laboratory experiments under simulated reservoir conditions. International Journal of Hydrogen Energy. 2018. Vol. 43. Iss. 45. N 20822-20835. DOI: 10.1016/j.ijhydene.2018.09.112
- Shi Z., Jessen K., Tsotsis T.T. Impacts of the subsurface storage of natural gas and hydrogen mixtures. International Journal of Hydrogen Energy. 2020. Vol. 45. Iss. 15, p. 8757-8773. DOI: 10.1016/j.ijhydene.2020.01.044
- Gogotsi Y., Portet C., Osswald S. et al. Importance of pore size in high-pressure hydrogen storage by porous carbons. International Journal of Hydrogen Energy. 2009. Vol. 34. Iss. 15, p. 6314-6319. DOI: 10.1016/j.ijhydene.2009.05.073
- Kashnikov Yu.A., Ashikhmin S.G., Gladyshev S.V., Popov S.N. Geomechanical and geodynamic problems accompanying the development of hydrocarbon fields. Journal of Mining Institute. 2010. Vol. 188, p. 153-157.
- Heinemann N., Alcalde J., Miocic J.M. et al. Enabling large-scale hydrogen storage in porous media – the scientific challenges. Energy & Environmental Science. 2021. Vol. 14. Iss. 2, p. 853-864. DOI: 10.1039/d0ee03536j
- Shevchuk S., Kvyatkovskaya S., Shevchuk R. Improving geodynamic monitoring practice in underground gas storage areas. 1st International Scientific Conference “Problems in Geomechanics of Highly Compressed Rock and Rock Massifs”, 15-22 July 2019, Vladivostok, Russia. E3S Web of Conferences. 2019. Vol. 129. N 01006. DOI: 10.1051/e3sconf/201912901006
- Vorobev S.V., Senderov S.M., Edelev A.V. Problem of appearance of excess gas in the Russian gas transportation network at short-time violations of Russian gas exports and the ways of solution. Proceedings of the Russian academy of sciences. Power Engineering. 2017. N 2, p. 151-164.
- Garaishin A.S., Ruban G.N. The basic criteria of a choice of a layer-accumulator for a burial place of industrial drains Karashursky UGS. Georesursy. 2010. N 4 (36), p. 26-29.
- Koshevarov P.A. Karashurskoe UGS – a reserve complex in Udmurtia. Gas industry. 2004. N 3, p. 20-21.
- Berta M., Dethlefsen F., Ebert M. et al. Geochemical Effects of Millimolar Hydrogen Concentrations in Groundwater: An Experimental Study in the Context of Subsurface Hydrogen Storage. Environmental Science and Technology. 2018. Vol. 52. Iss. 8, p. 4937-4949. DOI: 10.1021/acs.est.7b05467
- Hassannayebi N., Azizmohammadi S., de Lucia M., Ott H. Underground hydrogen storage: application of geochemical mo-delling in a case study in the Molasse Basin, Upper Austria. Environmental Earth Sciences. 2019. Vol. 78. Iss 5. N 177. DOI: 10.1007/s12665-019-8184-5
- Zhukov V.S., Kuzmin Y.O. Experimental evaluation of compressibility coefficients for fractures and intergranular pores of an oil and gas reservoir. Journal of Mining Institute. 2021. Vol. 251, p. 658-666. DOI:10.31897/PMI.2021.5.5
- Thanh To, Chandong Chang. Comparison of Different Permeability Models for Production-induced Compaction in Sandstone Reservoir. The Journal of Engineering Geology. 2019. Vol. 29. Iss. 4, p. 367-381. DOI: 10.9720/kseg.2019.4.367
- Pettersen O. Compaction, Permeability, and Fluid Flow in Brent-Type Reservoirs Under Depletion and Pressure Blowdown. The Open Petroleum Engineering Journal. 2010. N 3, p. 1-13. DOI: 10.2174/1874834101003010001
