Scientific and technical substantiation of the possibility for the organization of needle coke production in Russia
- 1 — Ph.D. Executive Director Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
- 2 — Ph.D. Researcher Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus
- 3 — Ph.D., Dr.Sci. Director Saint Petersburg Mining University ▪ Orcid ▪ Elibrary ▪ Scopus ▪ ResearcherID
Abstract
Russia is one of the world's leading steel producers, while about 33 % of production comes from the scrap remelted in arc steelmaking furnaces. The graphitized electrodes of SHP and UHP grades, mainly consisting of needle coke, are used for high current loads and temperatures in furnaces. USA, Japan, Korea, and China are focused on needle coke production, where coal (tar and pitch) and petroleum (decantoil), by-products of metallurgical factories and oil refineries, are used as raw materials. Russia's annual demand for needle coke is approximately 100 thousand tons, but all of it is covered by imports. Russia's raw material potential, established by the authors of the article, is more than 5 million tons per year and includes decantoil, coal tar and pitch, and heavy pyrolysis tar. The results of obtaining needle coke from decantoil and heavy pyrolysis tar are given below. The prototypes of needle coke were produced on specially designed delayed coking laboratory units (loading up to 0.25 and 80 kg). Raw materials were modified according to the original technology of Saint Petersburg Mining University, the convergence of target properties of which is confirmed by the results of quality analysis of the obtained needle coke, including after 100-fold scaling. The electrodes were molded from the obtained coke. After standardized stages of firing, mechanical processing and graphitization at 2,800-3,000 °C, the coefficient of linear thermal expansion was less than 1 × 10–6 К–1, and the value of specific electrical resistance was 7.1-7.4 μOhm, which proves that the obtained carbon material corresponds in quality to Japanese analogues and Super Premium needle coke.
Introduction
Needle coke is a purposely structured carbon material with a high proportion of graphite phase used for the manufacture of SHP (super-high power) and UHP (ultra-high power) graphitized electrodes for high and ultra-high power arc steelmaking furnaces [1-3]. Graphitized electrodes are the main material in the production of steel in arc steelmaking furnaces (consumption is about 5 kg per 1 ton of steel), which are about 85 % needle coke [4, 5]. Due to its oriented structure only such carbon material is able to withstand high current loads (current density up to 35 A/cm2) and temperatures up to 2,800 °C, generated in the end part of the electrode in the process of steel scrap remelting [6-8]. In the past two decades, with the development of alternative fuel transportation, the application of needle coke as a raw material for lithium-ion batteries of electric vehicles has been increasing [9]. However, the defining need for needle coke for seven decades has been driven primarily by the production of high-grade steel. According to the World Steel Association, in 2021 around 560 million tons (28.9 %) of steel in the world is produced in arc steelmaking furnaces (in Russia and CIS countries – about 33 %).
Needle coke is industrially produced from two types of raw materials – petroleum and coal. The oil raw material is decanted heavy catalytic cracking gas oil (decantoil) that is produced at refineries as a by-product of the production of the high-octane component of motor gasoline at catalytic cracking units (e.g., FCC) [10]. Needle coke was first commercially produced from oil raw material in the 1950s in the USA (Great Lakes Carbon Corporation). Coal raw material is a coal pitch purified from quinoline insoluble impurities, which is produced at coke plants of metallurgical plants as a by-product [11, 12]. Needle coke was produced for the first time from coal raw material in the 1970s in Japan (Mitsubishi Chemical) (Fig.1).
The global needle coke market is continuously growing (from USD 3.54 billion in 2017 to USD 4.15 billion in 2021). In 2022, manufacturing capacity is represented by 23 companies, seven of which are located in three countries – three in the US, three in Japan and one in Korea, the rest are in China (Table 1). Such a market for needle coke producers was established by the 1980s, and no new capacity was introduced after that (except in China). However, the raw material base and permanent growth of needle coke demand, driven by leadership in steel output (1.03 billion tons in 2021), allowed China to increase its actual production to 44 % of the world total (about 1.8 million tons per year) over the last 10 years. At the same time, the power shortage is a constraint [13, 14], which does not allow to commission the completed capacities of Chinese enterprises to produce 2.8 times more needle coke.
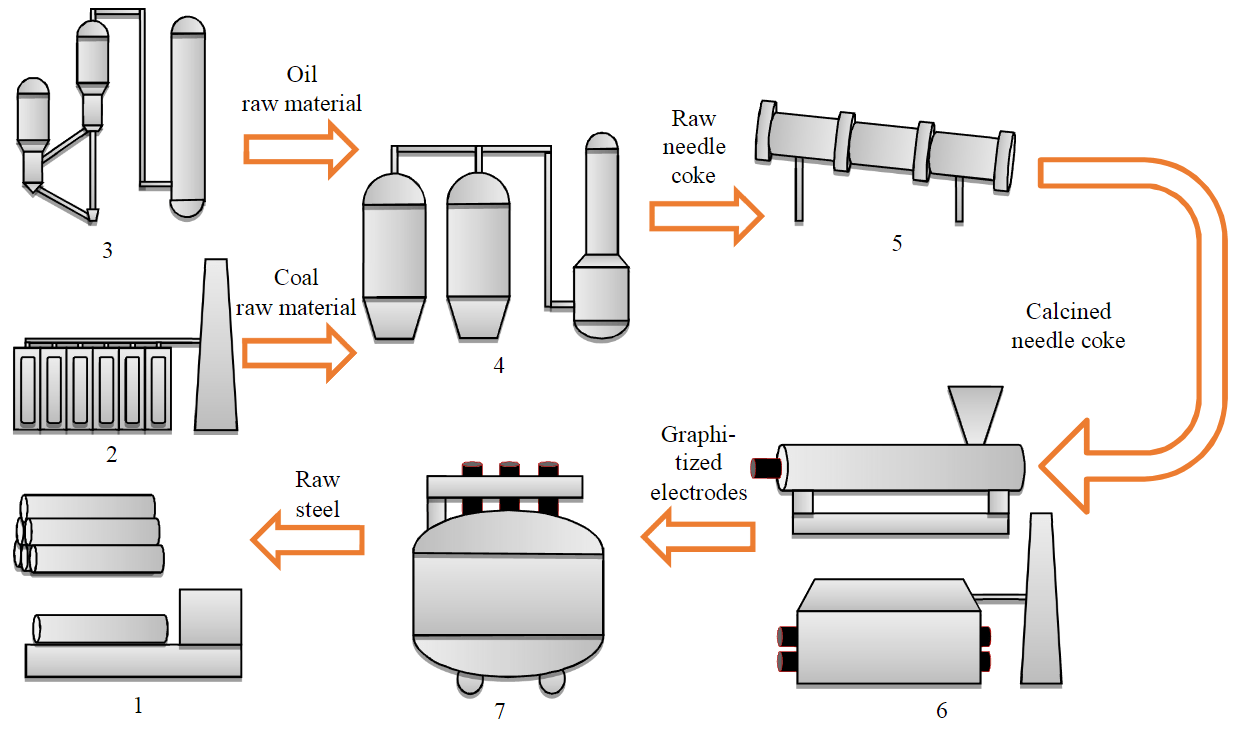
Fig.1. Block diagram of the complete cycle of needle coke production and application 1 – production of steel products; 2 – coke and chemical shop; 3 – oil refinery plant;
Table 1
World production of needle coke, thousand tons
|
Country |
Company |
Raw material type |
2019 |
2021 |
|
USA |
Asbury Carbons |
Oil |
No data |
No data |
|
GrafTech International |
Oil |
140 |
140 |
|
|
Phillips 66 Company |
Oil |
370 |
450 |
|
|
Japan |
Mitsubishi Chemical Holdings Corporation |
Coal |
80 |
80 |
|
Nippon Steel Chemical & Material Co. Ltd. (including C-Chem) |
Oil |
90 |
95 |
|
|
Coal |
110 |
110 |
||
|
Sumitomo Corporation |
Oil |
70 |
70 |
|
|
Korea |
Posco Chemical |
Coal |
50 |
63 |
|
Total |
910 |
1,008 |
||
|
China* |
16 companies in 2021 15 companies in 2019 |
Oil |
195 (360) |
501 (1,180) |
|
Coal |
250 (610) |
261 (1,000) |
||
|
Total worldwide |
1,355 |
1,770 |
||
* For China, production capacity is indicated in parentheses.
Along with China, the leading steel producing countries – India (118.2 million tons), Russia (75.6 million tons) and Iran (28.5 million tons), which are ranked (in 2021) second, fifth and tenth in the world respectively – are striving to start needle coke production, mainly to meet their domestic needs. Russia's need for needle coke to meet the needs of its own steelmaking enterprises is approximately 100 thousand tons per year. The Russian company OOO El 6 (formerly Energoprom) is a monopolist in the production of graphitized electrodes of SHP and UHP grades, which annually consumes up to 40 thousand tons of imported calcined needle coke.
The constantly growing demand and high market price of needle coke, which varies from 1,500 to 3,500 dollars per ton (2019-2022) with energy consumption for processing of one ton of raw material in the delayed coking process of 27-40 kWh, makes the organization of industrial production of needle coke not only strategically important, but also economically feasible. The volume of investments allocated for the implementation of projects on organization of oil needle coke production by India (Indian Oil Corporation Ltd., Paradip Refinery), Russia (PAO Gazprom Neft, Omsk Refinery) and Iran (Shazand Petrochemical Company, Imam Khomeini Shazand Oil Refinery) amounts to 150, 74-100 and 312 million dollars respectively. At the same time, the coke production volumes planned as a result of the projects' implementation are 56, 38 and 90 thousand tons per year for India, Russia and Iran respectively.
The prospect of organization of needle coke production in Russia, in addition to economic and strategic factors, as well as for other objects of mineral and fuel and energy complex, is determined by the availability of raw material resource base [15-17]. The assessment of raw material potential of Russia showed that it is represented both by traditional types of raw materials – decantoil [18] and coal pitch (together with coal tar) [19, 20], and promising one, not typical for the world market – heavy tar of natural gas pyrolysis (Fig.2).
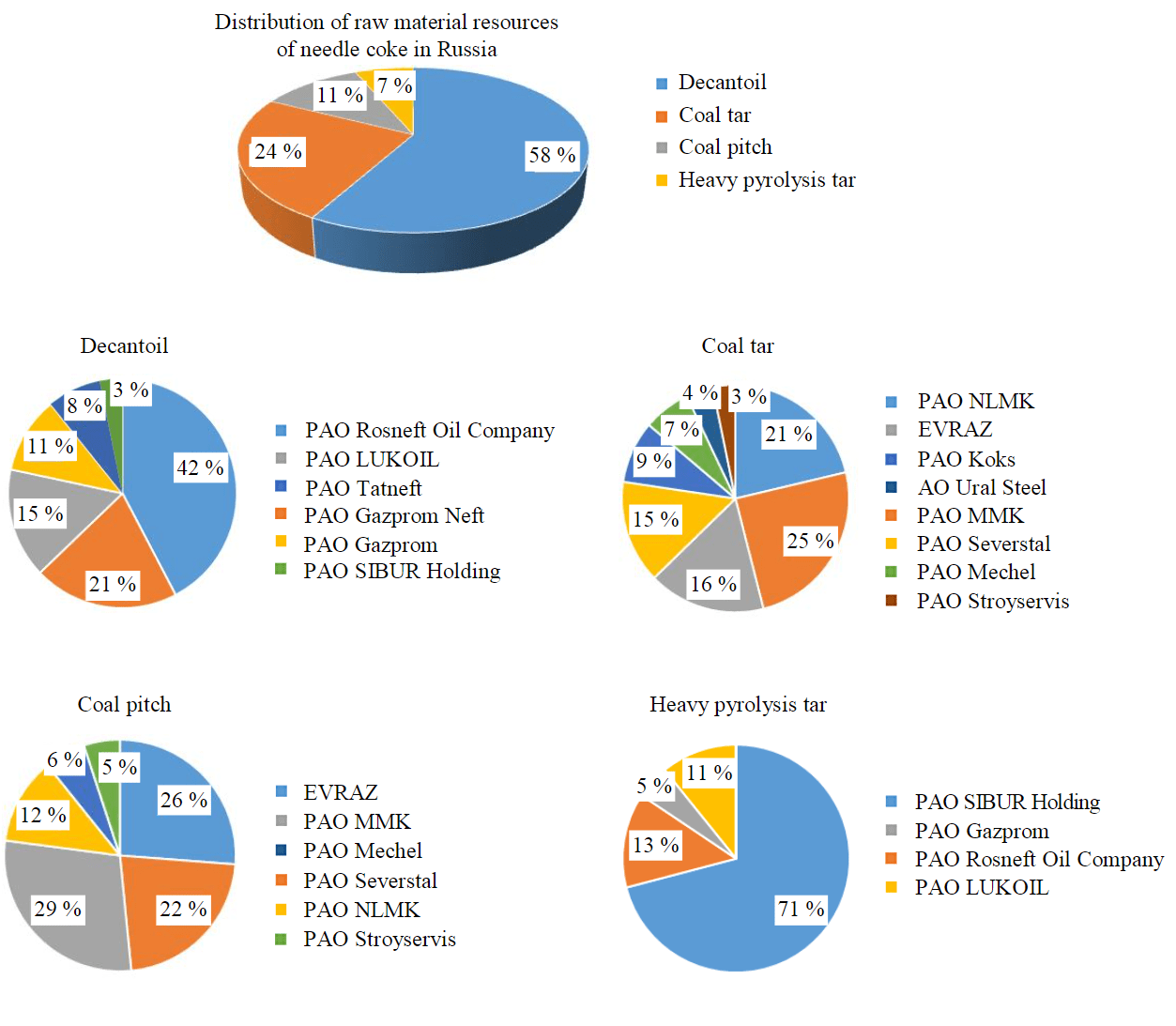
Fig.2. Assessment of raw material potential for needle coke production in Russia
Russia's raw material potential is represented by 48 technogenic sources and totals more than 5 million tons per year. Theoretically speaking, this amount is sufficient to produce up to 800,000 tons of needle coke per year, with Rosneft, Gazprom Neft, LUKOIL, Tatneft, Gazprom, NLMK, PAO SIBUR Holding, MMK, and EVRAZ taking the leading positions in terms of the volume of raw materials produced. However, most of this raw material cannot be directly utilized to produce needle coke in the delayed coking process. Differentiation by hydrocarbon composition, sulfur content and quinoline insoluble substances requires special preparation or modification of raw material. The world practice of technology implementation shows that for each raw material source it is necessary to develop an individual scientific and technical solution to obtain a high quality product [21-23], while the state regulation should be implemented in relation to the objects of mineral and raw material complex [24, 25].
The purpose of this work is to develop experimentally substantiated scientific and technical solutions for the creation of industrial production of needle coke in Russia.
Methods
Optimization of coking process and analysis of product quality
The research me-thodology of needle coke and electrodes obtained on its basis is developed at Saint Petersburg Mining University and is based on the knowledge of international [26, 27] and domestic scientific schools [28, 29].
It is necessary to note the significant contribution of scientists of the Ufa State Petroleum Technical University and the Institute of Petrochemical Refining of the Republic of Bashkortostan, the representatives of which back in the 1980s conducted pilot tests on the production of needle coke at the Krasnovodsk refinery (Turkmenistan) and the Novo-Ufimsky refinery, and currently provide scientific and technical support for the project of PAO Gazprom Neft at the Omsk refinery [30]. At Saint Petersburg Mining University, scientific work related to needle coke began in the mid-2010s under the leadership of V.Yu.Bazhin and N.K.Kondrasheva [31, 32], although the formation of the scientific school of carbon-graphite compounds was started in the middle of the 20th century by M.B.Rapoport.
To perform experimental studies on physical modeling of the process of needle coke production in the Scientific Center “Issues of Processing Mineral and Technogenic Resources” of Saint Petersburg Mining University two laboratory units of delayed coking with different volumes of raw material loading were specially developed (Fig.3).
The main task of the units is to select thermobaric conditions of the technological process and to produce laboratory (pilot) samples of products to evaluate their quality indicators. The basic process flow scheme for both designed units is identical. UZK-1 unit with 0.25 kg loading is used for kinetic studies and establishment of optimal mode of coking process, and LUZK unit with loading up to 80 kg is used for scaling of the process and development of pilot batch of needle coke of high quality.
The liquid phase raw material (base or modified) is placed in the reactor in a volume not exceeding 2/3 of the reactor to minimize the possibility of overflow during the process. Then the system is sealed, pressurized with inert gas (nitrogen), after which the raw material is heated to the temperature of coking mass (490-500 °C) for 12 h and kept at the set temperature to complete the process of obtaining raw needle coke. The pressure in the system is autogenous and is controlled by venting the generated gas-liquid products during delayed coking to the value of 0.35 MPa (ev.). The flow rate of waste hydrocarbon gases is monitored using a gas flow meter, and the mass of liquid distillates formed is recorded at the end of the experiment. After switching off the heating and complete cooling of the reactor, mechanical unloading of raw needle coke is performed. Then, the mass of carbon material obtained is recorded and the material balance of the process is summarized.
An obligatory stage of obtaining commercial needle coke is its calcination at temperatures from 1,100 to 1,500 °C, at which the removal of the remaining volatile substances and partial desulfurization occur and the degree of graphitization increases [33]. The calcination is carried out for one hour in a Nabertherm LTH 08/17 high-temperature muffle furnace.

Fig.3. Delayed coking laboratory units: a – UZK-1 (up to 0.25 kg); b – LUZK (up to 80 kg)
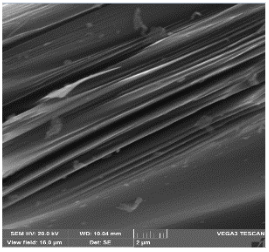
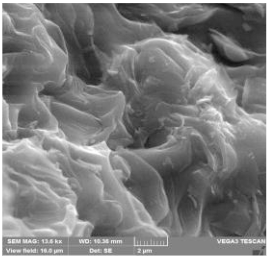
The surface morphology of needle coke was studied by scanning electron microscopy (SEM) on a Tescan Vega 3 LMH. Images were obtained in secondary electron (SE) in Resolution scan mode, field of view 16, 66 μm and 1 mm. The accelerating voltage was 20 kV and the emission current was 120 μA. Coke samples were identified and described according to the nomenclature.
Evaluation of needle coke microstructure by optical microscopy was carried out according to GOST 26132-84 “Petroleum and pitch cokes. Microstructure evaluation method” on the m-Vizio-MET-222 device [34, 35].
The crystal structure of needle coke was investigated on a Shimadzu XRD-7000 X-ray powder diffractometer (CuKα radiation 2.7 kW) at room temperature. Radiographs were taken with a long accumulation time (2 s) and a scanning step of 0.02°. From the obtained peaks with the maximum at the doubled Bragg diffraction angles 2θ, the interplanar distances by the value of diffraction maxima (002) and (100) as well as the average crystallite height Lc and the average diameter of hexagonal layers La were determined [28, 36].
Raman spectroscopy of needle coke samples was performed on a Renishaw inVia Raman Microscope with an excitation wavelength of 780 nm and using a 50-fold objective; the spectrum accumulation time was 10 s and each sample was imaged five times. The obtained Raman spectra (CR spectra) were normalized (by 100 units) and decomposed into five characteristic peaks (D1, D2, D3, D4, G) by Lorentz transformation.
Determination of sulfur content was performed on a Shimadzu XRF-1800 sequential wave X-ray fluorescence spectrometer. The apparatus is equipped with a 3.6 kW Rh anode X-ray tube; cathode current is 90 mA; tube voltage is 40 kV. The analysis was performed without prior ashing of the samples using the additive method (Ca in the form of CaCl2 was added) [37].
The coefficient of linear thermal expansion (CTE) of calcined needle coke samples was determined on a Netzsch DIL 402 C dilatometer at temperatures from 40 to 500 °C with a heating rate of 5 °C/min and blowing with an air stream (flow rate of 50 ml/min).
The effect of raw materials and coking conditions on the quality indicators of needle coke and properties of graphitized electrodes, which determine the necessary sequence of research and methods used, is presented in Fig.4.
Two types of base raw material, oil and gas, decantoil and heavy pyrolysis tar (HTP) respectively, were taken as the study material. A thermoplastic polymer of aromatic series was used as a modifier of the base raw material.
Fabrication of electrodes in a blind die
After analyzing the quality of the obtained needle coke on the UZK-1 unit, the primary assessment of the possibility of forming a carbon coke electrode from it by pressing in a blind die was carried out. The electrodes were prepared by mixing initial calcined needle coke with different proportion of medium-temperature pitch. The amount of pitch varied from 12 to 14 % by weight.
Mixing of needle coke with medium-temperature pitch (corresponding to GOST 10200-2017 grade B1) was carried out at elevated temperature and constant stirring (Fig.5) necessary to ensure effective distribution of binder throughout the volume of coke charge and pore filling [38], and included the following stages:
- mixing of needle coke with medium-temperature pitch; two fractions of needle coke were used – less than 0.5 mm (50 %) and 0.5-1.0 mm fraction – 50 %;
- heating of the obtained mixture to a temperature of 125 °C under constant stirring;
- mixing of needle coke with pitch for 40 min at a temperature of 125 °С (Fig.5, a, b);
- pressing of the obtained composite mixture at a temperature of 125 °C for 10 min under a pressure of 300 kg/cm2;
- extrusion of blanks – tablets with a diameter of 3.2 cm and a height of 6.2 cm.

Fig.4. Influence of raw materials and coking process conditions on quality indicators of needle coke and properties of graphitized electrodes
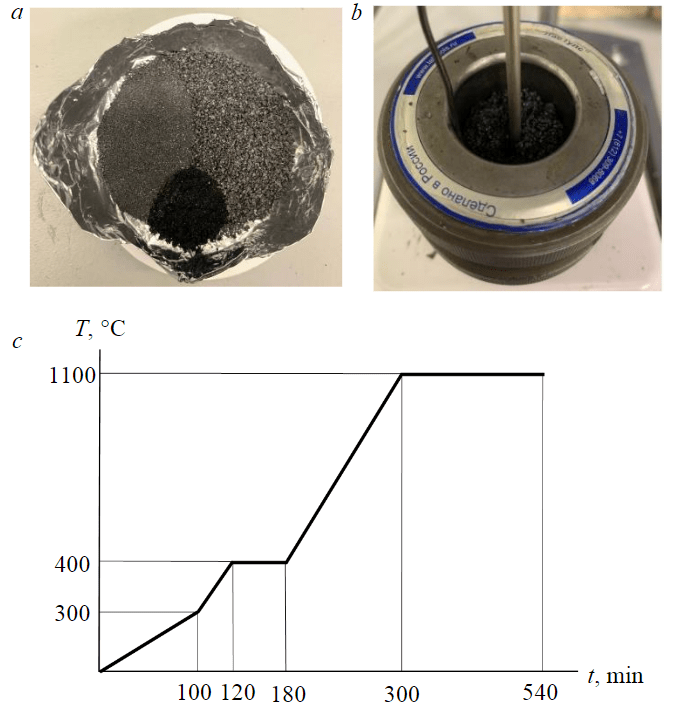
Fig.5. Preparation of annealed carbon coke electrode: a – charge for composite; b – hot mixing for molding; c – temperature mode of composite firing
The obtained blanks were further subjected to high-temperature firing in a layer of coke to create a reducing environment and reduce the burnup fraction of the calcined electrode. The maximum firing temperature was 1,100 °C with a total firing time of 540 min, isothermal holding was carried out at 1,100 °C for 240 min (Fig.5, c).
Manufacturing of electrodes by extrusion
Extrusion was used to evaluate the feasibility of forming electrodes from calcined needle coke samples obtained from an 80 kg LUZK unit. The process mode, raw material type, and modification method were preliminarily established at UZK-1. Manufacturing of the electrode by extrusion included the following steps:
- charge dosage according to the requirements for nipples at OOO El 6;
- mixing of coke fractions with heating, addition of electrode medium-temperature pitch to the heated carbon mass and mixing again;
- hot pressing of the obtained mass in an extruder;
- cutting of extrudates for subsequent firing and machining;
- firing of the obtained extrudates at 850 °C, isothermal holding at 850 °C was 1 h;
- mechanical processing of the obtained annealed electrodes;
- graphitization of annealed electrodes at a temperature of 2,800-3,000 °C.
Discussion of results
Preliminary assessment of the possibility of using the base raw material for needle coke production was determined by experimental results of hydrocarbon composition (SARA analysis) [39] and basic physical and chemical properties (Table 2).
Table 2
Physical and chemical properties and composition of basic coking raw material
|
Indicator |
Decantoil |
HTP |
Requirements of the Mining University |
|
Saturates, wt.% |
8.00 |
8.00 |
8.0-28.0 |
|
Aromatic, wt.% |
88.00 |
53.46 |
Min 61.4 |
|
Resins, wt.% |
4.00 |
24.14 |
1.0-14.0 |
|
Asphaltenes, wt.% |
0.10 |
14.54 |
Max 2.8 |
|
Density at 15 °C, kg/m3 |
1,046.1 |
1,073.3 |
– |
|
Sulfur content, wt.% |
0.142 |
0.086 |
Max 0.7 |
|
Ash content, wt.% |
0.012 |
0.010 |
0.0013-0.018 |
|
Dynamic viscosity (at 50 °C and different polymer concentration), mPa·s at 0 wt.% |
130.8 |
61.2 |
– |
|
at 5 wt.% |
1,000.0 |
301.6 |
– |
|
at 10 wt.% |
7,350.0 |
2,533.0 |
– |
|
at 15 wt.% |
98,200.0 |
10,107.0 |
– |
The comparative analysis of physical and chemical properties showed that the composition of the decantoil under study fully meets the requirements for needle coke raw material.
In order to select the optimal thermobaric conditions and modifier for the base raw material, experimental coking tests were initially carried out on the laboratory unit UZK-1 with a raw material loading of 0.25 kg. The coking mode was selected based on the capabilities of existing industrial delayed coking units – on the thermal load on the tube furnace and reactor, as well as on the maximum allowable pressure in adiabatic reactors (Table 3).
The obtained carbon samples (green coke) were calcined at 1,250-1,300 °C in a nitrogen current for one hour to evaluate the morphology of the obtained coke. Physical and chemical properties of calcined needle coke, parameters of its crystal lattice and coefficient of linear thermal expansion are presented in Table 4.
Linear dimensions and interplanar distances correspond to the values of needle coke samples obtained in the world. A slight increase in the interplanar distance d002 (3.4822-3.5161 Å) is observed with increasing modifier fraction in the raw material with a maximum at 10 wt.%. The obtained diffractogram shows a symmetrical shape of peak (002), which indicates the formation of a structure approaching the graphite one. According to the results of X-ray phase analysis, the character of the microstructure of petroleum coke can be judged by the ratio of the average height Lc and the average diameter La of crystallites. Thus, the further the value of this ratio is from unity, the more elongated is the character of the unit cell. When the modifier concentration increases from 0 to 10 wt.%, the ratio of Lc to La decreases, moving away from unity, and at 15 % it increases significantly (Table 4).
Table 3
Material balances of coking at the UZK-1 unit with 0.25 kg loading
|
Experiment number |
Taken, wt.% |
Received, wt.% |
|||||||
|
Base raw materials |
Modifier |
Total raw materials |
Coke |
Coking distillates |
Gas and losses |
Total products |
|||
|
Decantoil |
|
||||||||
|
1 |
100.0 |
0.0 |
100.0 |
44.8 |
36.2 |
19.0 |
100.0 |
|
|
|
2 |
97.5 |
2.5 |
100.0 |
46.0 |
36.6 |
17.4 |
100.0 |
|
|
|
3 |
95.0 |
5.0 |
100.0 |
45.6 |
37.2 |
17.2 |
100.0 |
|
|
|
4 |
90.0 |
10.0 |
100.0 |
44.4 |
39.2 |
16.4 |
100.0 |
|
|
|
5 |
85.0 |
15.0 |
100.0 |
45.2 |
42.2 |
12.6 |
100.0 |
|
|
|
Heavy pyrolysis tar |
|
||||||||
|
6 |
100.0 |
0.0 |
100.0 |
28.4 |
56.4 |
15.2 |
100.0 |
|
|
|
7 |
90.0 |
10.0 |
100.0 |
29.6 |
63.6 |
6.8 |
100.0 |
|
|
|
8 |
85.0 |
15.0 |
100.0 |
30.0 |
66.0 |
4.0 |
100.0 |
|
|
Table 4
Results of physical and chemical properties determination and calculations of structural characteristics of synthesized calcined needle coke
|
Indicator |
Experiment number |
|||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
|
Microstructure score |
5.40 |
5.70 |
6.10 |
6.15 |
5.70 |
4.80 |
5.30 |
5.40 |
|
Actual density, g/cm3 |
2.09 |
2.12 |
2.13 |
2.14 |
2.14 |
2.06 |
2.10 |
2.12 |
|
Volatile yield, wt.% |
5.27 |
4.33 |
4.86 |
5.14 |
4.33 |
5.11 |
5.12 |
5.09 |
|
Ash content, % |
0.05 |
0.05 |
0.05 |
0.04 |
0.04 |
0.06 |
0.07 |
0.07 |
|
Moisture, % |
0.05 |
0.05 |
0.06 |
0.06 |
0.05 |
0.12 |
0.13 |
0.20 |
|
Sulfur content, wt.% |
0.18 |
0.15 |
0.15 |
0.14 |
0.32 |
0.11 |
0.11 |
0.11 |
|
X-ray structure analysis |
|
|
|
|
|
|
|
|
|
Reflex score (002): |
|
|
|
|
|
|
|
|
|
2θ, degrees |
25.56 |
25.54 |
25.45 |
25.31 |
25.49 |
– |
– |
– |
|
β, degrees |
2.24 |
2.23 |
2.42 |
2.37 |
2.20 |
– |
– |
– |
|
d002, Å |
3.48 |
3.48 |
3.49 |
3.51 |
3.49 |
– |
– |
– |
|
Lc, Å |
36.03 |
36.19 |
33.33 |
34.03 |
36.68 |
– |
– |
– |
|
Reflex score (100): |
|
|
|
|
|
|
|
|
|
2θ, degrees |
43.04 |
42.88 |
42.69 |
42.76 |
42.94 |
– |
– |
– |
|
β, degrees |
2.23 |
2.21 |
2.11 |
1.97 |
2.24 |
– |
– |
– |
|
d100, Å |
2.09 |
2.10 |
2.12 |
2.11 |
2.10 |
– |
– |
– |
|
La, Å |
78.44 |
77.35 |
82.82 |
88.76 |
78.06 |
– |
– |
– |
|
La/Lc |
0.45 |
0.45 |
0.40 |
0.38 |
0.46 |
– |
– |
– |
|
CTE index (ASTM D 6745) at different temperature values, 1 ×10–6 K–1: |
|
|
|
|
|
|
|
|
|
at 40 °C |
25.09 |
– |
24.45 |
7.33 |
–10.43 |
– |
– |
– |
|
at 200 °C |
4.81 |
– |
14.00 |
14.14 |
11.87 |
– |
– |
– |
|
at 300 °C |
–19.20 |
– |
8.92 |
5.73 |
6.95 |
– |
– |
– |
|
at 400 °C |
10.51 |
– |
12.51 |
8.15 |
11.32 |
– |
– |
– |
|
at 500 °C |
4.35 |
– |
5.31 |
5.14 |
9.58 |
– |
– |
– |
The main indicator determining the quality of needle coke is the coefficient of linear thermal expansion; as a result of the tests performed, the tendency of decreasing the degree of deformation of carbon material under thermal influence with increasing the proportion of modifier in the initial raw material is observed (see Table 4). The minimum CTE 5.73 × 10–6 К–1 (at 300 °C) corresponds to a modifier concentration of 10 wt.%.
Since the long existence of the plastic state of the liquid phase and its low viscosity are necessary for the formation of the needle-like structure, the extreme dependence can be explained by a significant increase in the viscosity of the system due to the addition of a large concentration of the modifier – 15 wt.% (see Table 2). The limiting stage for liquid-phase thermolysis is diffusion, which is hampered by a significant increase in the viscosity of the system, while deterioration of growth and development of mesophase due to the termination of coalescence of mesophase spheres and inhibition of deformation of these spheres by convective flows are observed. Thus, a highly viscous medium is unfavorable for the formation of a striated structure. For confirmation, the dynamic viscosity of the mixture at 50 °C of decantoil with different concentrations of modifier was determined (see Table 2).
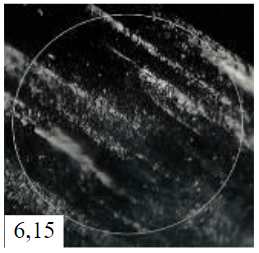
The combined study of physical and chemical properties and morphology of calcined coke by scanning electron microscopy and spectroscopy in plane polarized light (microstructure score) allowed to identify the best quality samples from both types of basic raw materials at its polymer modification: for decantoil it is experiment 4 with addition of 10 wt.% of modifier, and for heavy pyrolysis tar it is experiment 8 with addition of 15 wt.% of modifier. The most characteristic morphology for synthesized calcined needle coke with the highest microstructure score for the two types of raw materials is presented in Table 5. However, the samples obtained from HTP have a lower microstructure score and density, in morphology they represent a transitional structure with a relatively high proportion of isotropic phase. Therefore, further studies on needle coke production were carried out using only decantoil as a base raw material.
Raman spectroscopy of the needle coke sample obtained in experiment 4 (Fig.6) was carried out to quantify the fraction of graphitic phase and hard-to-graphite amorphous phase. Based on the results of integration of the obtained peaks, their areas were calculated (D1 = 1,312.53; D2 = 1,609.00; D3 = 1,498.00; D4 = 1,154.77; G = 1,584.31) and semi-quantitative estimation of the fraction of each phase in the sample of the calcined needle coke under study from experiment 4 was performed. The sample consists mainly of a misoriented graphite phase – 69.33 wt.%, which is typical for the turbostratic structure of needle coke, while the proportion of the ideal graphite phase is 6.10 wt.%, and of the amorphous phase – 24.59 wt.%.
Table 5
Scanning electron microscopy
|
Experiment number |
Field of view, μm |
Microstructure score |
||
|
1000 |
66 |
16 |
||
|
4 |
|
|
|
 |
|
8 |
|
|
|
|
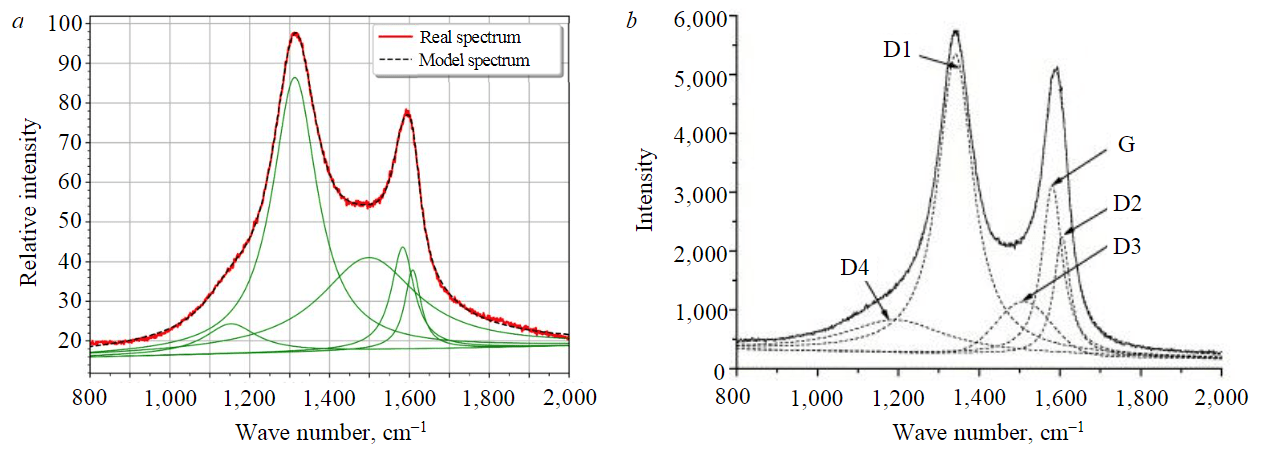
Fig.6. Decomposition of Raman spectra: a – for sample 4 under study; b – for carbon material D1 – disordered graphite lattice (edges of the graphene layer); D2 – disordered graphite lattice (surface layers of graphene); D3 – amorphous carbon (Lorentzian line shape); D4 – disordered graphite lattice; G – ideal graphite lattice
To clarify the convergence and reliability of the established optimal parameters of the coking process and to establish the possibility of realizing these data on an enlarged scale, the studies were carried out on the LUZK unit with simultaneous loading of up to 80 kg, which corresponded to 100-fold scaling (for each reactor). Decantoil with a modifier, which content in the base raw material was 10 wt.%, was used as base raw material. Two tests were conducted at LUZK to produce pilot batches of coke – without application of the modifier (experiment 9) and with its application (experiment 10). Material balances of the scaled pilot tests are presented in Table 6.
Table 6
Material balances of coking process scaling at LUZK unit
|
Substance |
Experiment 9 |
Experiment 10 |
||
|
Mass, g |
Content, wt.% |
Mass, g |
Content, wt.% |
|
|
|
Inflow |
|||
|
Decantoil |
20,900.00 |
100.00 |
19,140.00 |
90.00 |
|
Modifier |
0.00 |
0.00 |
2,126.10 |
10.00 |
|
Total |
20,900.00 |
100.00 |
21,266.10 |
100.00 |
|
|
Yield |
|||
|
Amount of distillates |
10,880.00 |
52.06 |
11,460.00 |
53.89 |
|
Needle coke |
7,060.00 |
33.78 |
7,460.00 |
35.08 |
|
Hydrocarbon gas |
2,419.00 |
11.57 |
2,126.00 |
10.00 |
|
Losses |
541.00 |
2.59 |
220.10 |
1.03 |
|
Total |
20,900.00 |
100.00 |
21,266.10 |
100.00 |
The analysis of the obtained results showed that at large-scale increase of the volume of one-time loading, the main technological parameters of the process do not go beyond the assumed corridor of the experiment error, in addition, there is a noticeable convergence of the product quality with the values of analogs of premium quality. At the same time, the yield of carbon material decreased and the yield of distillates increased. The change of material balance is caused by higher decomposition depth at thermolysis of small volume of raw materials and, consequently, higher coke yield.
At the comparative analysis of material balances of thermolysis of modified and unmodified raw material, a slight increase in yield of carbon material, distillate fractions and decrease in the share of gaseous fraction due to the increase in molecular weight of raw material due to the introduction of modifier is observed.
Needle coke samples obtained during experiments 9 and 10 were subjected to calcination in a nitrogen current at 1,400 °C for one hour with subsequent evaluation of the morphology of the modified sample by scanning electron microscopy [40, 41]. According to the results of evaluation of the obtained SEM images, it is possible to note the presence of a developed lamellar structure of the modified sample (experiment 10), while the total field of view practically does not include the isotropic amorphous phase, which is characteristic of high-quality needle coke.
Further study of the needle coke samples of basic and modified raw materials obtained during pilot tests at the LUZK unit was carried out on the basis of the engineering laboratory of OOO El 6 (Novocherkassk) to assess the quality of batches by the main consumer in Russia (Table 7).
Table 7
Results of research of physical and chemical properties of needle coke at OOO El 6
|
Indicator |
Method |
Needle coke |
Imported needle coke (Japan) |
|
|
Unmodified (experiment 9) |
Modified (experiment 10) |
|||
|
Actual density, g/cm3 |
GOST 22898 |
2.14 |
2.14 |
Min 2.13 |
|
Mass fraction of ash, % |
GOST 22692 |
0.2 |
0.2 |
Max 0.3 |
|
Mass fraction of total sulfur, % |
GOST 32465 |
0.12 |
0.05 |
Max 0.40 |
|
Mass fraction of nitrogen, % |
GOST 32979 |
0.24 |
0.22 |
Max 0.40 |
|
Microstructure score |
GOST 26132 |
5.3 |
5.8 |
– |
|
CTE 20-520 °C on an annealed electrode, 1 × 10–6 К–1 |
GOST R 54253 |
2.08 |
1.96 |
– |
According to the main physical and chemical properties, needle coke based on modified raw material is superior to the sample from basic decantoil, especially in terms of microstructure score and CTE, which indicates a greater degree of structurization of needle coke from modified raw material and also confirms the work of the modifier.
To obtain reliable results in practice, the estimation of CTE and specific electrical resistance of needle coke is carried out on a prepared graphitized electrode. Electrode samples were made from the worked needle coke and then sent for graphitization. The obtained samples of raw and annealed mechanically treated electrodes are presented in Fig.7, a, b, and samples of electrodes after graphitization at temperatures of 2,800-3,000 °C are presented in Fig.7, c.
Based on the results of the study of the obtained graphitized electrodes, the main quality indicator – coefficient of linear thermal expansion – for the sample from needle coke obtained by modification of raw materials with thermoplastic polymer takes lower values and meets the requirements for premium grades (CTE < 1 × 10–6 К–1). The required degree of graphitization of the two obtained electrode samples was estimated by the specific electrical resistance value, which was 7.1-7.4 μOhm × m and met the requirements for the electrodes of high and ultra-high power arc steelmaking furnaces.

Fig.7. Samples of electrodes manufactured by extrusion: a – raw electrodes; b – annealed and mechanically processed electrodes; c – graphitized electrodes
Conclusion
The following results were achieved during experimental studies on needle coke production at Saint Petersburg Mining University:
- The analytical assessment of raw material resources necessary for organization of industrial production of needle coke in Russia has been performed – a total of 48 technogenic sources: decantoil (58 %), heavy pyrolysis tar (7 %), coal tar (24 %) and coal pitch (11 %). Together, this amounts to more than 5 million tons per year, which is theoretically enough to produce up to 800,000 tons of needle coke annually. However, requirements to hydrocarbon composition, sulfur content and quinoline insoluble substances necessitate special preparation or modification of raw material and necessarily reduce the amount of practically possible needle coke production.
- A unique laboratory complex including two delayed coking units – UZK-1 (loading up to 0.25 kg) and LUZK (loading up to 80 kg) was created on the basis of the Scientific Center “Issues of Processing Mineral and Technogenic Resources” of Saint Petersburg Mining University, allowing to establish the influence of raw materials and technological parameters of the process on the quality indicators of needle coke and properties of graphitized electrodes.
- The best base hydrocarbon raw material for polymer modification is decantoil with its low content of sulfur (up to 0.7 %) and asphaltenes (not more than 2.8 %) as well as high content of aromatic hydrocarbons (more than 66 %).
- Heavy pyrolysis tar is a potentially more promising base raw material for needle coke production in Russia than decantoil, as it is practically free of sulfur, and no additional technological operations are required in the production chain to achieve this indicator. However, the needle coke from heavy pyrolysis tar, just like the one from decantoil, also requires pre-polymer modification to increase its structurization.
- It is established that the decomposition of Raman spectra is potentially the most informative method for estimating the degree of structurization of needle coke, since it allows quantifying the fractions of ideal graphite and amorphous phases. This type of analysis can be used in laboratories alongside microstructure score or can replace it, being less time-consuming.
- A needle coke raw material modifier, a thermoplastic polymer of aromatic series, has been experimentally proven to work for both decantoil and heavy pyrolysis tar. This is confirmed by 100-fold process scaling (based on CTE and microstructure score).
- It is established that the developed samples of needle coke after raw material modification in charge composition are well formed in the process of electrode manufacturing by extrusion and after standardized procedures of firing, mechanical treatment and graphitization at temperatures of 2,800-3,000 °C meet the requirements for this type of products (CTE < 1 ×10–6 К–1 and specific electrical resistance 7.1-7.4 μOhm).
- Highly structured needle coke obtained by the original technology of Saint Petersburg Mining University using 10 wt.% of modifier has an increased microstructure score of 5.8, compared to 5.3 without modification (on an enlarged batch of LUZK), lamellar structure with a low proportion of amorphous phase, low value of CTE of the electrode 0.897 ×10–6 К–1 (at the evaluation temperature of 20-520 °С), which corresponds to the quality of imported Japanese analogs and needle coke of Super Premium brand.
References
- Xian Xu, Louwei Cui, Junhe Shi et al. Effects of co-carbonization of medium and low temperature refined pitch and high temperature refined pitch on the structure and properties of needle coke. Journal of Analytical and Applied Pyrolysis. 2023. Vol. 169. N 105783. DOI: 10.1016/j.jaap.2022.105783
- Yangyang Yu, Feng Wang, Wiafe Biney B. et al. Co-carbonization of ethylene tar and fluid catalytic cracking decant oil: Development of high-quality needle coke feedstock. Fuel. 2022. Vol. 322. N 124170. DOI: 10.1016/j.fuel.2022.124170
- He Liu, Shouhui Jiao, Wenchao Liu et al. Effects of decompressing carbonization on the preparation of needle coke from FCC decant oil. Journal of Analytical and Applied Pyrolysis. 2022. Vol. 162. N 105454. DOI: 10.1016/j.jaap.2022.105454
- Kieush L., Schenk J., Koveria A. et al. Utilization of Renewable Carbon in Electric Arc Furnace-Based Steel Production: Comparative Evaluation of Properties of Conventional and Non-Conventional Carbon-Bearing Sources. Metals. 2023. Vol. 13. Iss. 4. N 722. DOI: 10.3390/met13040722
- Bazhin V.Yu., Krylov K.A., Sharikov F.Yu. Substantiation of thermophysical action over electrode paste to achieve an even structure of electrodes of needle coke for thermal furnaces. iPolytech Journal. 2023. Vol. 27. N 1, p. 161-173 (in Russian). DOI: 10.21285/1814-3520-2023-1-161-173
- Simakov A.S., Trifonova M.E., Gorlenkov D.V. Virtual Analyzer of the Voltage and Current Spectrum of the Electric Arc in Electric Arc Furnaces. Russian Metallurgy (Metally). 2021. Vol. 2021. N 6, p. 713-719. DOI: 10.1134/S0036029521060252
- Polyakov A.A., Gorlanov E.S., Mushihin E.A. Analytical Modeling of Current and Potential Distribution over Carbon and Low-Consumable Anodes during Aluminum Reduction Process. Journal of The Electrochemical Society. 2022. Vol. 169. Iss. 5. N 053502. DOI: 10.1149/1945-7111/ac6a16
- Sverguzova S.V., Sapronova Z.A., Zubkova O.S. et al. Electric steelmaking dust as a raw material for coagulant production. Journal of Mining Institute. 2023. Vol. 260, p. 279-288. DOI: 10.31897/PMI.2023.23
- Xiangye Li, Lei Zhao, Tieshi He et al. Highly conductive, hierarchical porous ultra-fine carbon fibers derived from polyacrylonitrile/polymethylmethacrylate/needle coke as binder-free electrodes for high-performance supercapacitors. Journal of Power Sources. 2022. Vol. 521. N 230943. DOI: 10.1016/j.jpowsour.2021.230943
- Stratiev D., Shishkova I., Yankov V. et al. Impact of H-Oil vacuum residue hydrocracking severity on fluid catalytic cracking unit performance. Petroleum Science and Technology. 2020. Vol. 38. Iss. 6, p. 565-573. DOI: 10.1080/10916466.2020.1772821
- Qi Li, Dongyun Han, Haiyan Qiao et al. Structural evolution of the thermal conversion products of modified coal tar pitch. Carbon Letters. 2023. Vol. 33. Iss. 1, p. 261-271. DOI: 10.1007/s42823-022-00433-8
- Malzahn J., Preciado I., Ding Wang et al. Effect of secondary gas-phase reactions (SGR) in pyrolysis of carbon feedstocks for anisotropic carbon materials production – 1: Controlling SGR to modify intermediate coal tar species to improve pitch anisotropy. Journal of Analytical and Applied Pyrolysis. 2022. Vol. 164. N 105541. DOI: 10.1016/j.jaap.2022.105541
- Bo Wang, Limao Wang, Shuai Zhong et al. Low-Carbon Transformation of Electric System against Power Shortage in China: Policy Optimization. Energies. 2022. Vol. 15. Iss. 4. N 1574. DOI: 10.3390/en15041574
- Guilhot L. An analysis of China’s energy policy from 1981 to 2020: Transitioning towards to a diversified and low-carbon energy system. Energy Policy. 2022. Vol. 162. N 112806. DOI: 10.1016/j.enpol.2022.112806
- Kapustin V.M., Glagoleva V.F. Physicochemical Aspects of Petroleum Coke Formation (Review). Petroleum Chemistry. 2016. Vol. 56. N 1, p. 1-9. DOI: 10.1134/S0965544116010035
- Litvinova T.E., Suchkov D.V. Comprehensive approach to the utilisation of technogenic waste from the mineral resource complex. Mining Informational and Analytical Bulletin. 2022. N 6-1, p. 331-348 (in Russian). DOI: 10.25018/0236_1493_2022_61_0_331
- Cherepovitsyn A., Rutenko E. Strategic Planning of Oil and Gas Companies: The Decarbonization Transition. Energies. 2022. Vol. 15. Iss. 17. N 6163. DOI: 10.3390/en15176163
- Lei Li, Xiongchao Lin, Yukun Zhang et al. Characteristics of the mesophase and needle coke derived from the blended coal tar and biomass tar pitch. Journal of Analytical and Applied Pyrolysis. 2020. Vol. 150. N 104889. DOI: 10.1016/j.jaap.2020.104889
- Kozlov A.P., Cherkasova T.G., Frolov S.V. et al. Innovative Coal-Tar Products at PAO Koks. Coke and Chemistry. 2020. Vol. 63. N 7, p. 344-350. DOI: 10.3103/S1068364X20070054
- Zhichen Zhang, Bin Lou, Ning Zhao et al. Co-carbonization behavior of the blended heavy oil and low temperature coal tar for the preparation of needle coke. Fuel. 2021. Vol. 302. N 121139. DOI: 10.1016/j.fuel.2021.121139
- Litvinenko V.S. Digital Economy as a Factor in the Technological Development of the Mineral Sector. Natural Resources Research. 2020. Vol. 29. N 3, p. 1521-1541. DOI: 10.1007/s11053-019-09568-4
- Smol M., Marcinek P., Duda J., Szołdrowska D. Importance of Sustainable Mineral Resource Management in Implementing the Circular Economy (CE) Model and the European Green Deal Strategy. Resources. 2020. Vol. 9. Iss. 5. N 55. DOI: 10.3390/resources9050055
- Ershov M.A., Savelenko V.D., Makhmudova A.E. et al. Technological Potential Analysis and Vacant Technology Forecasting in Properties and Composition of Low-Sulfur Marine Fuel Oil (VLSFO and ULSFO) Bunkered in Key World Ports. Journal of Marine Science and Engineering. 2022. Vol. 10. Iss. 12. N 1828. DOI: 10.3390/jmse10121828
- Litvinenko V.S., Petrov E.I., Vasilevskaya D.V. et al. Assessment of the role of the state in the management of mineral resources. Journal of Mining Institute. 2023. Vol. 259, p. 95-111. DOI: 10.31897/PMI.2022.100
- Litvinenko V., Bowbriсk I., Naumov I., Zaitseva Z. Global guidelines and requirements for professional competencies of natural resource extraction engineers: Implications for ESG principles and sustainable development goals. Journal of Cleaner Production. 2022. Vol. 338. N 130530. DOI: 10.1016/j.jclepro.2022.130530
- Andreoli S., Brown S.H., Uppili S., Eser S. Laboratory Coking and a New Optical Texture Classification Parameter: Screening of Petroleum Refinery Streams for Premium Needle Coke Production. Energy & Fuels. 2022. Vol. 36. Iss. 18, p. 10910-10919. DOI: 10.1021/acs.energyfuels.2c02077
- Zhichen Zhang, Hui Du, Shuhai Guo et al. Probing the effect of molecular structure and compositions in extracted oil on the characteristics of needle coke. Fuel. 2021. Vol. 301. N 120984. DOI: 10.1016/j.fuel.2021.120984
- Ismagilov Z.R., Sozinov S.A., Popova A.N., Zaporin V.P. Structural Analysis of Needle Coke. Coke and Chemistry. 2019. Vol. 62. N 4, p. 135-142. DOI: 10.3103/S1068364X19040021
- Dolomatov M.Y., Burangulov D.Z., Dolomatova M.M. et al. Low-Sulphur Vacuum Gasoil of Western Siberia Oil: The Impact of Its Structural and Chemical Features on the Properties of the Produced Needle Coke. Journal of Carbon Research. 2022. Vol. 8. Iss. 1. N 19. DOI: 10.3390/c8010019
- Zaporin V.P., Sukhov S.V., Fedotov K.V. et al. Patent N 2717815 RF. Method of producing oil needle coke. Publ. 25.03.2020. Bull. N 9.
- Bazhin V.Yu. Structural Modification of Petroleum Needle Coke by Adding Lithium on Calcining. Coke and Chemistry. 2015. Vol. 58. N 4, p. 138-142. DOI: 10.3103/S1068364X15040043
- Kondrasheva N.K., Vasilev V.V., Boitsova A.A. Study of Feasibility of Producing High-Quality Petroleum Coke from Heavy Yarega Oil. Chemistry and Technology of Fuels and Oils. 2017. Vol. 52. N 6, p. 663-669. DOI: 10.1007/s10553-017-0758-x
- Sharikov Yu.V., Sharikov F.Yu., Krylov K.A. Mathematical Model of Optimum Control for Petroleum Coke Production in a Rotary Tube Kiln. Theoretical Foundations of Chemical Engineering. 2021. Vol. 55. N 4, p. 711-719. DOI: 10.1134/S0040579521030192
- Nazarenko M.Yu., Saltykova S.N. Obtaining semi-coke from oil shale of the Leningrad deposit. Chernye metally. 2022. N 10, p. 4-8 (in Russian). DOI: 10.17580/chm.2022.10.01
- Vinogradova A., Gogolinskii K., Umanskii A. et al. Method of the Mechanical Properties Evaluation of Polyethylene Gas Pipelines with Portable Hardness Testers. Inventions. 2022. Vol. 7. Iss. 4. N 125. DOI: 10.3390/inventions7040125
- Sultanbekov R.R., Schipachev A.M. Manifestation of incompatibility of marine residual fuels: a method for determining compatibility, studying composition of fuels and sediment. Journal of Mining Institute. 2022. Vol. 257, p. 843-852. DOI: 10.31897/PMI.2022.56
- Sverchkov I.P., Gembitskaya I.M., Povarov V.G., Chukaeva M.A. Method of reference samples preparation for X-ray fluorescence analysis. Talanta. 2023. Vol. 252. N 123820. DOI: 10.1016/j.talanta.2022.123820
- Qingwen Wei, Keliang Pang, Cai Liang et al. The fracture characteristics and enhancement mechanism of crushing strength for the coke with hot pressing. Journal of Analytical and Applied Pyrolysis. 2023. Vol. 171. N 105981. DOI: 10.1016/j.jaap.2023.105981
- Povarov V.G., Efimov I.I. Use of the UNIFAC model in the calculation of physicochemical properties of ecotoxicants for technological and ecoanalytical purposes. Journal of Mining Institute. 2023. Vol. 260, p. 238-247. DOI: 10.31897/PMI.2023.41
- Savchenkov S.A., Bazhin V.Yu., Povarov V.G. Research on the process of gadolinium recovery from the melt of salts on formation of Mg – Zn – Gd master alloys for manufacturing of magnesium and aluminium special-purpose alloys. Non-ferrous Metals. 2020. N 1, p. 35-40. DOI: 10.17580/nfm.2020.01.06
- Savchenkov S.A., Bazhin V.Yu., Brichkin V.N. et al. Production Features of Magnesium-Neodymium Master Alloy Synthesis. Metallurgist. 2019. Vol. 63. N 3-4, p. 394-402. DOI: 10.1007/s11015-019-00835-6