Uranium in man-made carbonates on the territory of Ufa
- 1 — Ph.D. Academic Secretary Vernadsky State Geological Museum of the RAS ▪ Orcid
- 2 — Leading Ufa Federal Research Centre of the RAS ▪ Orcid
- 3 — Ph.D. Associate Professor Tomsk Polytechnic National Research University ▪ Orcid
- 4 — Ph.D. Senior Lecturer Bashkir State University ▪ Orcid
- 5 — Researcher Vernadsky State Geological Museum of the RAS ▪ Orcid
- 6 — Ph.D. Researcher CY Cergy Paris Université ▪ Orcid
- 7 — Ph.D., Dr.Sci. Chief Researcher Vernadsky State Geological Museum of the RAS ▪ Orcid
Abstract
The paper presents the results of analyzing uranium content in man-made carbonates (scale crusts) on the territory of Ufa based on examination of 42 samples. The median uranium content in the investigated samples stands at 1.44 mg/kg, which is significantly lower than the background values (scales from the Lake Baikal water, a clarke of sedimentary carbonate rocks) and data on other settlements of the Republic of Bashkortostan. Low values of uranium content are probably associated with the effects of the three leading factors, i.e. specific subsurface geology of the territory (gypsum, limestone); types of water supply; water treatment processes for the centralized type of water supply. Spatial distribution of uranium in man-made carbonates is characterized with uniformity, which is disturbed in two cases, i.e. a change of the water supply type (from centralized to individual); and material of the vessels used for boiling the water. No significant differences were detected when comparing samples of man-made carbonates associated with different sources of water supply (the bucket and infiltration types of water intake) and the types of household filters.
Introduction
Water is one of the main sources of chemical elements for the human body [1, 2]. Both excess and deficiency of these elements can have a negative impact on health, triggering a number of possible diseases [3-5].
The hazards of uranium result from a combination of three effects [6], i.e. its toxic, radioactive and carcinogenic action [7-9]. At the same time, it does not perform any known biological functions [6, 10, 11]. Also the daughter products of uranium decay, of which radon, a radioactive gas, is the best known [12-14], produce predominantly negative effects. Studies have shown that radon can cause lung cancer, leukemia and other oncological diseases [15-17].
When ingested with water in low concentrations, uranium does not cause any significant harm as a radionuclide, but it can affect the human body as a toxic chemical element [18]. A number of studies show that high background levels of uranium in drinking water can cause renal cell carcinoma, kidney failure and other diseases [6, 19, 20]. Therefore, geochemical investigations of potable water for excessive concentrations of chemical elements, including uranium, are an important field of geoecology, medical geology, and toxicology [6, 21, 22]. Identification of such anomalies at the regional and local levels can seriously contribute to the prevention of chronic diseases caused by environmental factors of both natural and man-made origin.
Studies conducted in several regions of the Russian Federation (the Irkutsk, Tomsk and Chelyabinsk Regions, the Altai and Buryat Republics) and Kazakhstan (the Pavlodar Region) have shown that one of the indirect indicators of potable water quality are man-made carbonates (scale) that are formed when water is boiled in households [23-26]. These studies show that, in terms of material composition, the scale formed in domestic water boiling consists mainly of different variations of calcium carbonate. X-ray powder diffractometry and scanning electron microscopy confirm this by indicating that the main mineral phases in the scale are calcite or aragonite [24, 25], while the content of other mineral phases ranges within first few percent. This is explained by the fact that mainly hydrocarbonate calcium and magnesium-calcium waters are used for potable water supply.
In natural conditions the uranium geochemistry is quite versatile [27] and depends on a number of physical and chemical parameters, i.e. pH, Eh, presence of complexing compounds (carbonates, phosphates, sulphates, etc.) [28-30]. Under oxidizing conditions, uranium is most commonly found in its hexavalent form (U6+) as uranyl ion (UO22+), which forms more stable complexes and has higher mobility in natural waters [30]. When carbonate ions are present, uranyl ions easily associate with them producing (UO2)(CO3)0, (UO2)(CO3)22−, UO2(CO3)34− compounds [31-33], and precipitates with basic carbonate minerals (calcite, aragonite, dolomite) [34-36]. Deposition of uranium from solutions is usually associated with higher evaporation rates or local oversaturation [33, 37], which is observed in the case of potable water boiling (combination of the thermodynamic and evaporation geochemical barriers).
At the same time, studies of parallel samples of water and man-made carbonates on the territory of the Tomsk and Pavlodar regions, as well as in the Baikal area show that there is a positive correlation between the uranium content in water and in the man-made carbonates (the linear correlation indicator varies from 0.62 to 0.96).
The purpose of this research is to analyze the content of uranium in man-made carbonates (potable water scales) on the territory of Ufa. This study is a logical continuation of works to assess the geo-ecological situation in the Republic of Bashkortostan from the standpoint of medical geo-logy [38].
Geologically, Ufa is located within the eastern margin of the East European Platform (the Russian Plate) (Fig.1). Most of the territory of the city is located on a flat plateau-like elevation with steep slopes, bounded on the west, south and east by the valleys of the Ufa and Belaya rivers, also known as the Ufa Peninsula. The Archean crystalline basement is overlain by a thick (about 8 km) layer of sedimentary rocks in this area. Permian, Neogene and Quaternary sediments are recorded within the city boundaries [39].
The Permian System within the territory of Ufa is represented with the Kungurian and Ufimian Stages of the lower Series. The Kungurian Stage includes light grey gypsum and anhydrite with interbeds of gypsiferous clays and dolomite, found at the base of steep riverbanks. The thickness of the sediments is up to 340 m. The Ufa Stage is represented with frequent interbedded limestones, clayey dolomites, marls, clays, siltstones and sandstones with an overall thickness ranging within 15-25 m and up to 60 m in some areas.
Neogene sediments are represented with the Kinel Formation in the valleys of the Belaya and Ufa Rivers, as well as the poorly defined Akchagyl and Apsheron Stages in the Belo-Ufimsk interfluve area. The Kinel Formation includes heavy clays, sands and gravels up to 70-100 m in total thickness. The Akchagyl and Apsheron Stages are composed of reddish-brown and greyish-brown heavy clays with interbands of sand in the lower part with a total thickness of 50 m [39].
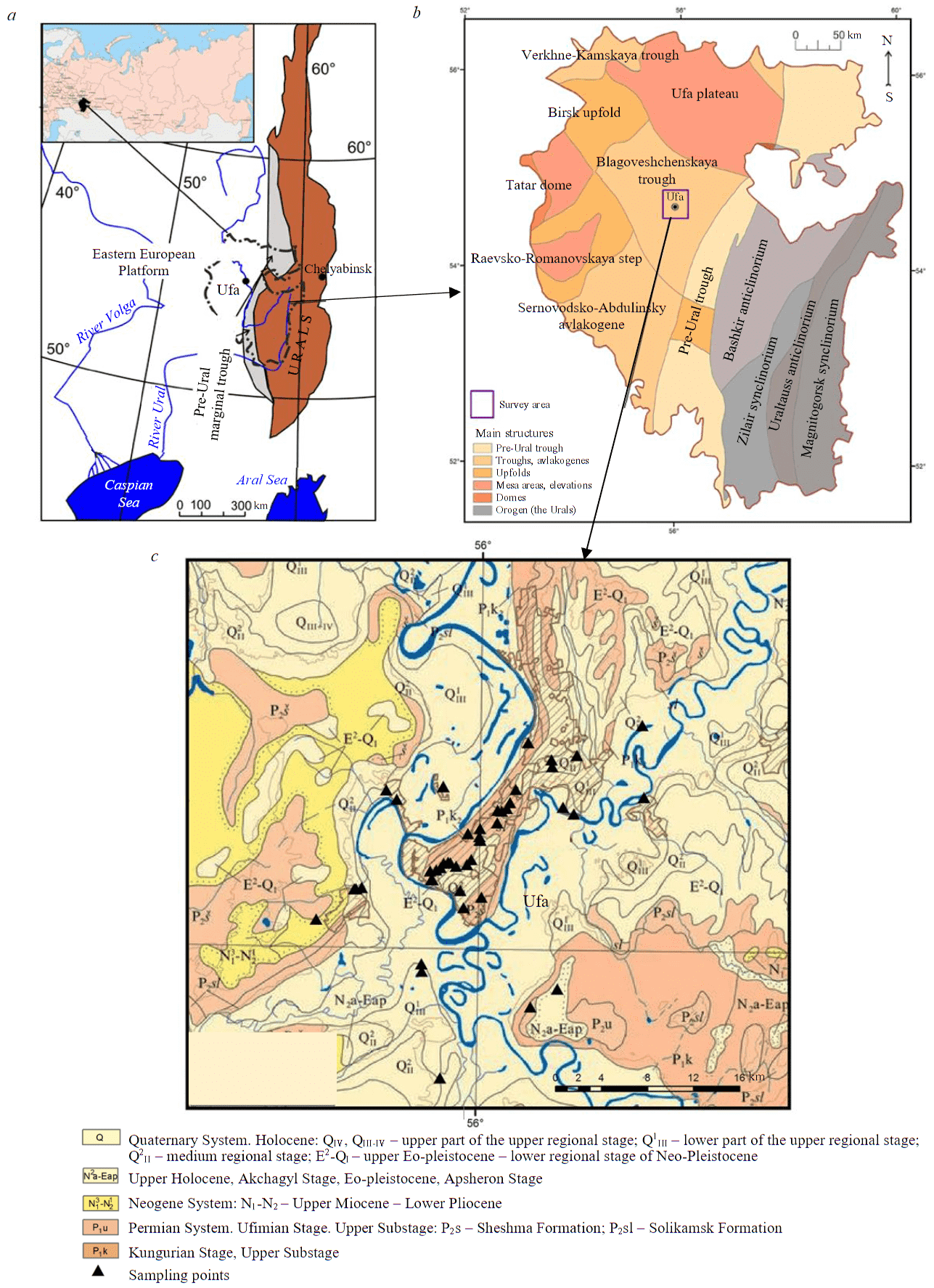
Fig.1. Subsurface geology of the survey area: a – location of the survey area; b – tectonic zoning of the Republic of Bashkortostan (State Geological Map, sheet N40(41), scale 1:1,000,000 (2001); c – a fragment of the geological map of the Republic of Bashkortostan with indication of sampling points, scale 1:500,000 (2011)
Quaternary sediments in the Belaya and Ufa river valleys are made up of alluvial gravels and sands with a thickness of 10-15 m overlain by periglacial clayey sediments (sandy loams, loams, clays) ranging from 1-3 to 15-20 m, while within the Belo-Ufa interfluve area the sediments are alluvial-deluvial (clays, loams) and their thickness varies from 0.5-2 to 10-15 m [39].
The city's drinking water supply is based on the waters of the Ufa River, as well as groundwater available both in the river valleys (Belaya, Ufa, Shugurovka) and in the interfluve area. According to research data, there exist noticeable differences in the groundwater composition in the southern and northern parts of the city. The chemical composition of groundwater in the southern (residential) part of the city is predominantly bicarbonate, sulphate-hydrocarbonate and hydrocarbonate-chloride magnesium-calcium, magnesium-calcium-sodium. The water salinity is 0.66-1.29 g/l, pH 7.2-7.62. The content of trace elements is below the maximum permissible concentration, and the main indicator of groundwater pollution in this part of the city is nitrogen compounds, as the concentration of nitrate-ion in wells of private houses reaches 1100-1530 mg/dm3. Some areas are noted to have excessive levels of Cr, Hg, petroleum products, phenols and other chemical elements and organic compounds [40].
In the northern (industrial) part of the city, the groundwater is sulphate-hydrocarbonate, hydrocarbonate-chloride and chloride-hydrocarbonate calcium, calcium-sodium, sodium-calcium, sodium-magnesium-calcium, ammonium-sodium. The type of water changes from sulphate-sodium to magnesium-chloride and calcium-chloride with salinity rising up to 6-11.5 g/dm3 [40]. The radiation safety indicators comply with the standards, i.e. the total α-radioactivity is below 0.02 Bq/L with the relevant standard level being 0.2 Bq/L, while the total β-radioactivity is less than 0.1 Bq/L with the standard of 1.0 Bq/L [41].
Water is supplied to households in Ufa by the Ufavodokanal State Unitary Enterprise. The territory of the city is provided with centralized water supply and sewerage systems, which comprises seven large-scale water intakes. i.e. an open river water intake (Northern, the bucket-type) and six infiltration-type water intakes with a total capacity of 613 thousand m3/day [41]. Potable water, supplied to the population, meets the requirements of the state standards [42].
Methods
Sampling
Data on the uranium content in 42 samples of salt sediments from potable water were analyzed within the city territory, 36 of which were taken from households with centralized water supply, and 6 from water wells and boreholes. A comparative analysis was made using data on the uranium content in samples of man-made carbonates collected in the territory of the Ufa district (28 samples from 18 settlements). Data available for other settlements in the Republic of Bashkortostan, mainly for the cities [43, 44], were used randomly. The samples were collected in conformity with the available recommendations [45]. The sampling was done from various vessels, i.e. enameled and electric kettles, pots, samovars, which are used for boiling water from both centralized and individual (water wells, boreholes) water supply sources. The following criteria were also considered while sampling: the time of scale formation, i.e. when the vessels were last decalcified before the sampling, the depth of the water supply source and whether a filter was used.
Studies carried out in different locations [23-25] have proved the efficiency of using the scale crust as an indirect indicator of the potable water quality. Correct sampling minimizes the impact of factors such as type of vessels and the time of scale formation. The leading factors in this case are the type and the source of water supply as well as the use of a filter.
Analytical methods
The uranium content in man-made carbonates was analyzed using the instrumental neutron activation analysis in the nuclear geochemical laboratory of the “Uranium Geo-logy” International Innovative Research and Education Center that uses the IRT-T nuclear research reactor at the Tomsk Polytechnic University (Accreditation Certificate N RA.RU.21АБ27 dated 08.04.2015, analysts: A.F.Sudyko, L.F.Bogutskaya).
X-ray powder diffractometry was used to analyze the material composition of the man-made carbonates. Seven randomly selected scale samples were analyzed using the X-ray phase analysis with the Bruker D2 Phaser X-ray diffractometer. The X-ray images were obtained in the Bragg – Brentano geometry. The following imaging parameters were used: anode material – Cu (copper), X-ray tube voltage – 30 kV, current – 10 mA, imaging angles 2Ɵ – from 10 to 70°, imaging step – 0.02°, exposure time – 1 s per point, rotation – 20 rpm. The obtained diffractograms were interpreted using the DIFFRAC.Eva and TOPAS software suites based on the PDF-2 X-ray powder diffractometry databases of International Center for Diffraction Data (ICDD, Denver, USA).
The data were processed with the Statistica 8.0 and Microsoft Excel 2016 software packages. Statistical processing of the data included calculation of the following parameters: arithmetic mean, standard error, geometric mean, median, mode, minimum and maximum values, standard deviation, coefficient of variation, skewness ratio, degree of excess and their standard errors. In data processing, values below the detection limit (0.1 mg/kg) were replaced by half the value (0.05 mg/kg). Since the nature of uranium distribution in the studied selection does not match normal distribution according to various tests (Kolmogorov – Smirnov, Lilliefors, chi-squared tests), the mean value was taken to equal the median calculated without taking into account the outstanding samples, although they are shown in the discussion of the results obtained. Geochemical specialization of the man-made carbonates and the patterns of uranium accumulation in them were determined by comparing with the background values, i.e. a clarke of sedimentary carbonate rocks – 2.2 mg/kg [46], scale from Lake Baikal water obtained by the authors – 4.51 mg/kg [47].
Maps of spatial distribution of natural radioactive elements were built using the ArcGIS 10.3 software in Spatial Analyst module with the Inverse Distance Weighted Interpolation method. The choice of interpolation method was based on a relatively small number of survey points and their uneven spatial distribution. The values were approximated as weighted averages within a certain distance. The nearest objects have a greater weight, while the distant ones have a relatively low influence on the calculations (the weights are inversely proportional to the measure of distance).
Discussion of results
Material composition of man-made carbonates
The performed diffractometry studies of seven randomly selected scale samples show that all the samples are made up of various calcium carbonate modifications: the dominant mineral in four samples is calcite, i.e. its rhombohedral modification, and in the remaining three samples the main mineral is aragonite in orthorhombic modification. These data agree well with previous studies of the man-made carbonates in other territories (the Tomsk, Irkutsk, Pavlodar Regions and the Republic of Buryatia) [23-25].
Based on analyzing 42 samples of man-made carbonates collected on the territory of Ufa, the median content of uranium is 1.44 mg/kg ranging from 0.05 (half the detection limit by the instrumental neutron activation analysis) up to 28.9 mg/kg. It should be noted that the distribution of uranium in the studied sample selection is relatively homogeneous, as values of the arithmetic mean (1.82 mg/kg), geometric mean (1.3 mg/kg) and median content are quite close. The coefficient of variation for this selection (N = 42) is 82 %. The median content is significantly lower than in the background values which are used in comparative analysis to study of the man-made carbonates, i.e. the scale crust from Lake Baikal water (4.51 mg/kg) [47] and sedimentary carbonate rocks (2.2 mg/kg) [46]. The median content of uranium in the man-made carbonates within the territory of Ufa is significantly lower than the average value for the Republic of Bashkortostan, i.e. 5.4 mg/kg [44], as well as in other studied areas: the Tomsk region (1.9 mg/kg) and the Pavlodar region (27.4 mg/kg), the Baikal region (21.1 mg/kg), the Altai Republic (10.7 mg/kg) [47].
The low content of uranium in the man-made carbonates may be due to the specific features of subsurface geology within the territory of the city, as most of the city is located in the areas of gypsum and limestone distribution. A similar trend was previously revealed on the Ufa plateau, located in the northern part of the Republic [43, 44]. This factor, i.e. the subsurface geology, is more evident when comparing the obtained results with the data on the uranium content in the man-made carbonates from other large settlements of the Republic of Bashkortostan, mainly from the cities (see Table 1).
Table 1
Uranium content in samples of man-made carbonates from various settlements
|
Settlement name |
Number of samples |
Content, mg/kg |
Rocks/soils encountered on the day surface |
|
Sibay |
4 |
6.21 |
D2kr2 – Karamalytash Formation. Upper Subformation. Dacites, rhyodacites, basalts and their lava-breccias, hyaloclastites and calc-sinters, andesite-basalts, silicate tuffites, cryolites, and jaspers (over 750 m); |
|
Sterlitamak |
4 |
6.88 |
N₂3ak+ap – Akchagyl and Apsheron Stages. Clays, siltstones, sands, pebbles; |
|
Oktyabrsky |
14 |
8.5 |
P1šš – Ufimian Stage. Sheshma Horizon. Sheshma Formation – sandstones, siltstones, pudding rocks, marls, limestones, dolomites, and gypsums; |
|
Kumertau |
12 |
9.3 |
T1 – pudding rocks, sandstones with bands of clays and siltstones; |
|
Akyar |
9 |
10.2 |
J1–2bm – Baymak Formation. Mica and carbonaceous clays, siltstones, clayey and mica sands, rare interbeds of brown coals, lenses of siderites; below this layer – sandy-gravely pebbles, carbonaceous clays with interbeds of coals, interbeds of brown-iron ores (30-270 m); |
|
Tuimazy |
7 |
11.9 |
P1šš – Ufimian Stage. Sheshma Horizon. Sheshma Formation – sandstones, siltstones, pudding rocks, marls, limestones, dolomites, and gypsums; |
|
Davlekanovo |
4 |
17.5 |
P1šš1 – Ufimian Stage. Sheshma Horizon. Lower Formation. Pudding rocks, sandstones, siltstones, argillites (Kamyshin layers); |
|
Akhunovo |
2 |
22.7 |
γδPz₃ – granodiorites and plagiogranites |
|
Chekmagush |
10 |
27.8 |
P1šš2 – Ufimian Stage. Sheshma Horizon. Middle Formation. Pudding rocks, sandstones, siltstones, argillites, marls, limestones (Buraev layers); |
The obtained data make it possible to conclude that the leading role in the variations of uranium content in the man-made carbonates is played by the specific features of subsurface geology on this territory. Thus, in the Davlekanovo and Chekmagush settlements the uranium concentration reached 17.5 and 27.8 mg/kg respectively, which may be due to uranium intake from the Lower Permian redbed sandstones, shallow crystalline basement, as well as the occurrence of oil fields in these areas, which contributes to the migration of uranium together with oil from deeper layers. A higher content of uranium (22.7 mg/kg) in the scale was also found in samples from the Akhunovo village, located on the Akhunovsky granite massif [43, 44].
When comparing data on the uranium content in the samples from Ufa and settlements of the Ufa district it should be noted that the samples collected in the district are characterized with a higher median content of uranium. Samples of the man-made carbonates were obtained in 18 settlements within the boundaries of the Ufa district. The uranium content varies from 1.2 (Mokrousovo) to 14.5 mg/kg (Nagaevo, Nikolaevka). The median content for samples from settlements in the Ufa district was 4.9 mg/kg, which is more than three times higher than the median concentration in samples from the city of Ufa.
One of the likely factors that explains such a big difference is the type of water supply: the vast majority of scale samples from the city of Ufa were collected in households with centralized water supply, while in the settlements of the Ufa district the samples were mostly collected in households with individual water supply (water wells, boreholes, springs, etc.). Availability of the centralized water supply significantly affects the use of various water treatment systems. Thus, at the Northern water intake the Ufavodokanal State Unitary Enterprise uses sorption treatment of water with powdered activated carbon, cyclic pressure-tank filters, high-rate filters with burnt rock as a locally produced filtering material [41].
The spatial distribution of uranium was assessed based on the results of defining the uranium content in samples collected on the territory of Ufa. Analysis of the schematic map in Fig.2 shows that there exists a homogeneous distribution of uranium in the man-made carbonates. Deviations were registered in individual points (southern part of the city, the left bank, the suburbs) where concentrations reach 29 mg/kg. These deviations are probably related to the following two factors: the material of the vessels where the water was boiled (Fig.2, location 1); the individual type of water supply (a well) (Fig.2, location 2). The probable impact of the vessel material is explained by the fact that within the same household, in addition to this particular sample that was collected for an all-metal kettle, three more samples were also obtained from three other kettles with the uranium content of 0.99; 1.25; and 1.31 mg/kg.
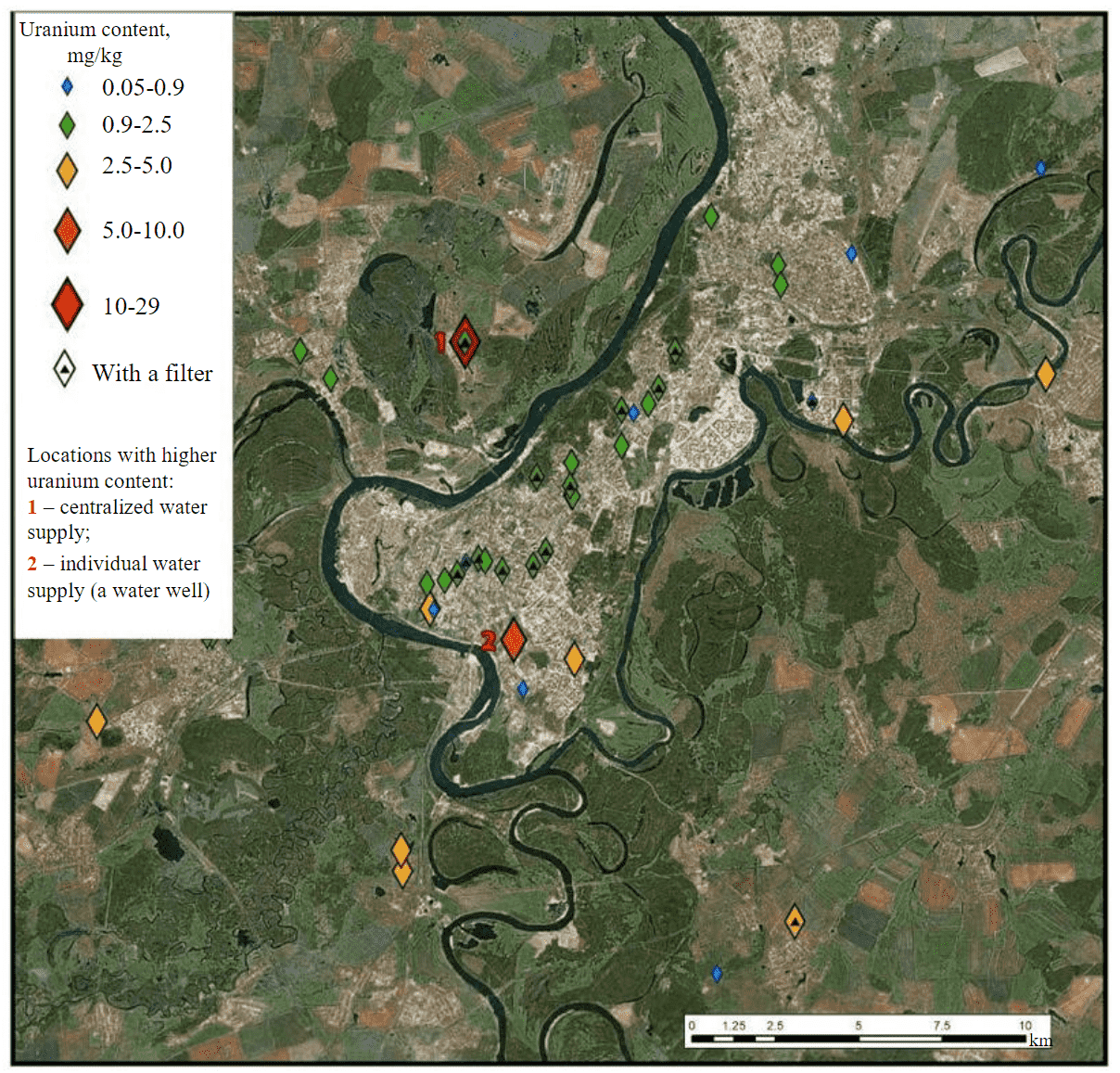
Fig.2. Spatial distribution of uranium content in samples of man-made carbonates collected in the city of Ufa
Data from 18 settlements in the suburban area of Ufa (Table 2) were taken for comparison. Individual water supply sources (water wells, boreholes, springs, etc.) are mainly developed within its boundaries. Analysis of the spatial distribution of uranium in the man-made carbonate samples in the territory of the Ufa district shows its heterogeneous nature (Fig.3). Halos of high concentrations of this chemical element are confined to the western and southwestern parts of the district (Bulgakovo, Dmitrievka, Marmylevo, Nikolaevka, Taptykovo, Nagaevo, Yumatovo), where the average content of uranium exceeds 10 mg/kg (Table 2).
In terms of their geological distribution within the territory of Ufa district, halos of the increased uranium content in the man-made carbonates are confined to the areas of loamy and clayey sediments (Upper Pliocene, Pleistocene, Holocene). A significant effect of the subsurface geology within this territory is explained by the fact that the vast majority of the man-made carbonate samples were obtained in households with individual water supply sources that do not typically have water treatment systems other than potable water filters (such as pitcher filters).
Effects of the water supply type (centralized/individual) and the filtering processes
Surveys conducted in the Tomsk and Pavlodar regions indicate that there exist significant differences regrading accumulation of chemical elements in the man-made carbonates depending on the type of the water supply source: in the case of the centralized water supply, concentrations of most chemical elements are significantly lower than those in water from the individual water supply sources [23, 24].

Fig.3. Spatial distribution of uranium content in samples of man-made carbonates collected in the territory of the Ufa district
Table 2
Uranium content in man-made carbonates from various settlements in the Ufa district
|
Settlement name |
Number |
Content, mg/kg |
Rocks/soils encountered on the day surface |
|
Maksimovka |
1 |
0.87 |
Q1III – Upper Quaternary, lower part. Loams, sandy loams, sands, pebbles |
|
Mokrousovo |
1 |
1.2 |
N2kn – Kinel Formation. Clays, sands, pebbles |
|
Chesnokovka |
1 |
2.2 |
P1ir – Kungurian Stage. Irensky Horizon. Anhydrites, gypsums, dolomites; |
|
Berezovka |
1 |
2.3 |
N2kn – Kinel Formation. Clays, sands, pebbles |
|
Mikhailovka |
2 |
2.9 |
P1u – Ufimian Stage. Limestones, marls, clays, siltstones, sandstones; |
|
Samokhvalovka |
1 |
3.4 |
Q1III – Upper Quaternary, lower part. Loams, sandy loams, sands, pebbles; |
|
Schmidtovo |
2 |
3.97 |
P1kg – Kungurian Stage. Poorly defined sediments. Gypsums, anhydrites, salts, |
|
Vavilovo |
1 |
5.2 |
N23-Q1 – Upper Pliocene – Lower Quaternary sediments. Sandy clays and loams with marl nodules, in some places with pebbles at the base |
|
Osorgino |
1 |
6.8 |
N₂3akk – Akchagyl Stage. Middle Substage. Akkulayevsky Horizon. Sands, siltstones, clays, pebbles |
|
8th of March |
4 |
8.1 |
Q1III – Upper Quaternary, lower part. Loams, sands, pebbles; |
|
Zhukovo |
4 |
8.5 |
P1šš1 – Ufimian Stage. Sheshma Horizon. Lower Formation. Pudding rocks, sandstones, siltstones, argillites (Kamyshin layers); |
|
Yumatovo |
1 |
10.4 |
P1šš1 – Ufimian Stage. Sheshma Horizon. Lower Formation. Pudding rocks, sandstones, siltstones, argillites (Kamyshin layers); |
|
Taptykovo |
1 |
13.4 |
P1sk – Ufimian Stage. Solikamsk Horizon. Argillites, siltstones gypsified, limestones, |
|
Dmitrievka |
1 |
13.6 |
N23-Q1 – Upper Pliocene – Lower Quaternary sediments. Sandy clays and loams with marl nodules, in some places with pebbles at the base; |
|
Bulgakovo |
1 |
14.0 |
N2kn – Kinel Formation. Clays, sands, pebbles |
|
Marmylevo |
1 |
14.1 |
P1u – Ufimian Stage. Limestones, marls, clays, siltstones, sandstones; |
|
Nagaevo |
3 |
14.5 |
N22+3 – Middle and Lower Pliocene combined. Pebbles, sands, clays, ferruginous pudding rocks |
|
Nikolaevka |
1 |
14.5 |
P1u – Ufimian Stage. Limestones, marls, clays, siltstones, sandstones |
For this study, the selected man-made carbonate samples from Ufa were divided into two groups depending on the type of water supply. The results obtained make it possible to claim that the centralized water supply systems significantly reduce the uranium content in the man-made carbonates (4.0 mg/kg with the individual water supply, and 1.4 mg/kg with the centralized water supply) (Table 3). Decrease of uranium concentration is related to the water treatment processes used by water services company including the Ufavodokanal State Unitary Enterprise (sorption treatment of water with powdered activated carbon, cyclic pressure-tank filters, high-rate filters with burnt rock as a locally produced filtering material).
Table 3
Uranium content in man-made carbonates on the territory of Ufa depending on the type of water supply and filtering processes
|
Type of water supply |
Number of samples |
Median content of uranium, mg/kg |
|
Centralized water supply: |
35 |
1.4 |
|
with a filter |
15 |
1.34 |
|
without a filter |
19 |
1.43 |
|
Individual water supply |
5 |
4.0 |
A wider introduction and development of the centralized type of water supply and, accordingly, methods of water pre-treatment (physical, chemical, physicochemical) can reduce not only the content of uranium, but also that of other chemical elements. It is especially important for rapidly developing urbanized areas, which include the territory of Ufa. This issue acquires a special significance in case the water supply source is confined to groundwater in quaternary sediments, as they are more vulnerable to man-made impact, including chemical, physical and biological contamination. This poses a serious threat to the health of the local population.
A possible impact of the original water source has been assessed. It is known that the water supply system of Ufa is based on the operation of eight water intakes (groundwater and river-type). The largest of them are the Southern water intake, with the maximum capacity of 240 thousand m3/day, and the Northern the bucket-type with 200 thousand m3/day. A total of 10 samples were collected to assess the possible impact of the original source of water supply: 5 in the northern part of the city and 5 in the southern part. The results turned out to be close in their values, i.e. 1.3 mg/kg in the north and 1.1 mg/kg in the south.
The study considers a possible influence of the household filtration process on the uranium content in the man-made carbonates: during the sampling the household owners were asked to provide information on the use of filters. A sufficient size is available only for samples collected from the centralized water supply: 15 samples with prefiltering prior to boiling (the Aquaphor, Barrier, and Geyser filter brands); 19 samples without prefiltering. The differences were minimal: 1.34 mg/kg for the samples where prefiltering was performed; 1.43 mg/kg for the samples without prefiltering. The study did not address the effect of the internal filtering material, as in each particular case the filter can be designed to entrap different substances.
Conclusion
The following conclusions were made based on the survey results:
- the average content of uranium in the man-made carbonates on the territory of Ufa is 1.44 mg/kg, which is three times lower than in the scale from the water of Lake Baikal, i.e. 4.51 mg/kg;
- the low values of uranium content in the scale are associated with the specific features of subsurface geology in the area, as most of the city is located within a distribution zone of gypsums and limestones;
- spatial distribution of uranium is described as homogeneous;
- significant differences were found between the uranium content in scale samples from the centralized (1.4 mg/kg) and the individual (4.0 mg/kg) types of water supply;
- no significant differences were revealed between the uranium content in the scale when comparing different sources of centralized water supply (the Northern bucket-type and the Southern infiltration water intakes) and the use of home filters.
The detected level of uranium content in the man-made carbonates on the territory of Ufa can be characterized as non-hazardous, since other investigations on the territory of the Pavlodar Region have shown that the maximum permissible concentration of uranium in water (0.015 mg/l according to SanPiN 1.2.3685-21) correlates with its content in the man-made carbonates (about 30 mg/kg) [24].
References
- Baranovskaya N.V., Rikhvanov L.P., Ignatova T.N. et al. Essays on Geochemistry. Tomsk: Tomskii politekhnicheskii universitet, 2015, p. 378 (in Russian).
- WHO. Guidelines for Drinking-water Quality: Fourth Edition Incorporating the First Addendum. Geneva: World Health Organization, 2017, p. 541.
- Patel A.I., Hecht C.E., Cradock A. et al. Drinking Water in the United States: Implications of Water Safety, Access, and Consumption. Annual Review of Nutrition. 2020. Vol. 40, p. 345-373. DOI: 10.1146/annurev-nutr-122319-035707
- McDonough L.K., Meredith K.T., Nikagolla C., Banati R.B. The influence of water-rock interactions on household well water in an area of high prevalence chronic kidney disease of unknown aetiology (CKDu). Npj Clean Water. 2021. Vol. 4. N 2. DOI: 10.1038/s41545-020-00092-0
- Miller J., Workman C.L., Panchang S.V. et al. Water Security and Nutrition: Current Knowledge and Research Opportunities. Advances in Nutrition. 2021. Vol. 12. Iss. 6, p. 2525-2539. DOI: 10.1093/advances/nmab075
- Bjørklund G., Semenova Y., Pivina L. et al. Uranium in drinking water: a public health threat. Archives of Toxicology. 2020. Vol. 94, p. 1551-1560. DOI: 10.1007/s00204-020-02676-8
- Keith S., Faroon O., Roney N. et al. Toxicological Profile for Uranium. Agency for Toxic Substances and Disease Registry (US). Atlanta: Agency for Toxic Substances and Disease Registry, 2013, p. 526.
- Bjørklund G., Christophersen O.A., Chirumbolo S. et al. Recent aspects of uranium toxicology in medical geology. Environmental Research. 2017. Vol. 156, p. 526-533. DOI: 10.1016/j.envres.2017.04.010
- Ning Gao, Zhihui Huang, Haiqiang Liu et al. Advances on the toxicity of uranium to different organisms. Chemosphere. 2019. Vol. 237. N 124548. DOI: 10.1016/j.chemosphere.2019.124548
- Konietzka R. Gastrointestinal absorption of uranium compounds – A review. Regulatory Toxicology and Pharmacology. 2015. Vol. 71. Iss. 1, p. 125-133. DOI: 10.1016/j.yrtph.2014.08.012
- Minghao Ma, Ruixia Wang, Lining Xu et al. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environment International. 2020. Vol. 145. N 106107. DOI: 10.1016/j.envint.2020.106107
- Vengosh A., Coyte R.M., Podgorski J., Johnson T.M. A critical review on the occurrence and distribution of the uranium- and thorium-decay nuclides and their effect on the quality of groundwater. Science of the Total Environment. 2022. Vol. 808. N 151914. DOI: 10.1016/j.scitotenv.2021.151914
- Darby S., Hill D., Auvinen A. et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. British Medical Journal. 2005. Vol. 330. DOI: 10.1136/bmj.38308.477650.63
- Kheifets L., Swanson J., Yuan Y. et al. Comparative analyses of studies of childhood leukemia and magnetic fields, radon and gamma radiation. Journal of Radiological Protection. 2017. Vol. 37. N 2, p. 459-491. DOI: 10.1088/1361-6498/aa5fc7
- UNSCEAR: sources, effects and risks of ionizing radiation UNSCEAR 2017. Report to the General Assembly Scientific Annexes A and B. New York: United Nations Publication, 2018. 184 p.
- Zlobina A.N., Rikhvanov L.P., Baranovskaya N.V. Radioecological Hazard for the Population Living in the Regions with High Radioactive Granites. Bulletin of the Tomsk Polytechnic University. Geo Assets Engineering. 2019. Vol. 330. N 3, p. 111-125 (in Russian). DOI: 10.18799/24131830/2019/3/172
- Maier A., Wiedemann J., Rapp F. et al. Radon Exposure – Therapeutic Effect and Cancer Risk. International Journal of Molecular Sciences. 2021. Vol. 22. Iss. 1. N 316. DOI: 10.3390/ijms22010316
- Sahoo S.K., Jha V.N., Patra A.C. et al. Scientific background and methodology adopted on derivation of regulatory limit for uranium in drinking water – A global perspective. Environmental Advances. 2020. Vol. 2. N 100020. DOI: 10.1016/j.envadv.2020.100020
- Zamora M.L., Tracy B.L., Zielinski J.M. et al. Chronic Ingestion of Uranium in Drinking Water: A Study of Kidney Bioeffects in Humans. Toxicological Sciences. 1998. Vol. 43. Iss. 1, p. 68-77. DOI: 10.1006/toxs.1998.2426
- Gandhi T.P., Sampath P.V., Maliyekkal S.M. A critical review of uranium contamination in groundwater: Treatment and sludge disposal. Science of the Total Environment. 2022. Vol. 825. N 153947. DOI: 10.1016/j.scitotenv.2022.153947
- Rikhvanov L.P., Baranovskaya N.V., Sudyko A.F. Chemical elements in the human body as the basis for implementation of medical geology concepts. Gornyi zhurnal. 2013. N 3, p. 37-42 (in Russian).
- Farkhutdinov I., Farkhutdinova L., Zlobina A. et al. Historical aspects of medical geology. Earth Sciences History. 2020. Vol. 39. Iss. 1, p. 172-183. DOI: 10.17704/1944-6187-39.1.172
- Mongolina Т.А., Baranovskaya N.V., Soktoev B.R. Ultimate composition of salt scales in potable waters of the Tomsk Region. Izvestiya Tomskogo politekhnicheskogo universiteta. 2011. Vol. 319. N 1, p. 204-211 (in Russian)
- Arynova Sh.Zh. Ultimate composition of salt formations from natural fresh waters as an indicator of ecological safety of water consumption: Avtoref. dis. … kand. geol.-mineral. nauk. Tomsk: Natsional'nyi issledovatel'skii Tomskii politekhnicheskii universitet, 2016, p. 22 (in Russian).
- Soktoev B.R., Rikhvanov L.P., Matveenko I.A. Mineralogical and geochemical characteristics of drinking water salt deposits. XIX International Scientific Symposium in honor of Academician M.A.Usov “Problems of Geology and Subsurface Development”, 6-10 April 2015, Tomsk, Russia. IOP Conference Series: Earth and Environmental Science. 2015. Vol. 27. N 012042. DOI: 10.1088/1755-1315/27/1/012042
- Winde F., Erasmus E., Geipel G. Uranium contaminated drinking water linked to leukaemia – Revisiting a case study from South Africa taking alternative exposure pathways into account. Science of the Total Environment. 2017. Vol. 574, p. 400-421. DOI: 10.1016/j.scitotenv.2016.09.035
- Evseeva L.S., Perel'man A.I., Ivanov K.E. Geochemistry of uranium in the hypergenesis zone. Moscow: Gosatomizdat, 1974, p.278 (in Russian).
- Langmuir D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochimica et Cosmochimica Acta. 1978. Vol. 42. Iss. 6, p. 547-569. DOI: 10.1016/0016-7037(78)90001-7
- Silva R.J., Nitsche H. Actinide environmental chemistry. Radiochimica Acta. 1995. Vol. 70-71, p. 377-396. DOI: 10.1524/ract.1995.7071.special-issue.377
- Mühr-Ebert E.L., Wagner F., Walther C. Speciation of uranium: Compilation of a thermodynamic database and its experimental evaluation using different analytical techniques. Applied Geochemistry. 2019. Vol. 100, p. 213-222. DOI: 10.1016/j.apgeochem.2018.10.006
- Cumberland S.A., Douglas G., Grice K., Moreau J.W. Uranium mobility in organic matter-rich sediments: A review of geological and geochemical processes. Earth-Science Reviews. 2016. Vol. 159, p. 160-185. DOI: 10.1016/j.earscirev.2016.05.010
- Geskeis H., Lützenkirchen J., Polly R. et al. Mineral-water interface reactions of actinides. Chemical Reviews. 2013. Vol. 113. Iss. 2, p. 1016-1062. DOI: 10.1021/cr300370h
- Gurzhiy V.V., Kalashnikova S.A., Kuporev I.V., Plášil J. Crystal chemistry and structural complexity of the uranyl carbonate minerals and synthetic compounds. Crystals. 2021. Vol. 11. Iss. 6. N 704. DOI: 10.3390/cryst11060704
- Elless M.P., Lee S.Y. Uranium solubility of carbonate-rich uranium-contaminated soils. Water, Air, and Soil Pollution. 1998. Vol. 107, p. 147-162. DOI: 10.1023/a:1004982515941
- Kelly S.D., Rasbury E.T., Chattopadhyay S. et al. Evidence of a stable uranyl site in ancient organic-rich calcite. Environmental Science and Technology. 2006. Vol. 40. Iss. 7, p. 2262-2268. DOI: 10.1021/es051970v
- Rihs S., Sturchio N.C., Orlandini K. et al. Interaction of uranyl with calcite in the presence of EDTA. Environmental Science and Technology. 2004. Vol. 38. Iss. 19, p. 5078-5086. DOI: 10.1021/es049847b
- Zhiwei Niu, Xiaoyan Wei, Shirong Qiang et al. Spectroscopic studies on U(VI) incorporation into CaCO3: Effects of aging time and U(VI) concentration. Chemosphere. 2019. Vol. 220, p. 1100-1107. DOI: 10.1016/j.chemosphere.2019.01.010
- Farkhutdinova L.M., Farkhutdinov I.M. The Republic of Bashkortostan as a Ground for Research in Medical Geology. Herald of the Academy of Sciences of the Republic of Bashkortostan. 2017. Vol. 23. N 2 (86), p. 83-92 (in Russian).
- Abdrakhmanov R.F., Buryachok O.V., Bakhtiyarov S.A. Formation of groundwaters in the city of Ufa. Geologicheskii sbornik. 2011. Vol. 9, p. 262-275 (in Russian).
- Abdrakhmanov R.F. Hydrogeochemistry of Urban Territories in the Southern Fore-Ural Areas. Geochemistry International. 2019. Vol. 57. N 7, p. 812-820. DOI: 10.1134/S0016702919070036741
- Quality of potable water. URL: https://www.ufavodokanal.ru/voda/water-quality (accessed 16.03.2021) (in Russian).
- Valeev T.K., Sulejmanov R.A., Egorova N.N. et al. The Hygienic Characteristic of Risk of Influence of Quality of Water on Health of the Population of Large Industrial Centre. Meditsina truda i ekologiya cheloveka. 2016. N 3, p. 11-17 (in Russian).
- Farkhutdinov I.M., Soktoev B.R., Rikhvanov L.P. Influence of Geological Factors on Uranium and Thorium Distribution in Drinking Water Salt Deposits (Republic Of Bashkortostan). Bulletin of the Tomsk polytechnic university. Geo assets engineering. 2020. Vol. 331. N 4, p. 16-27 (in Russian). DOI: 10.18799/24131830/2020/4/2590
- Farkhutdinov I., Soktoev B., Zlobina A. et al. Influences of geological factors on the distribution of uranium in drinking water limescale in the junction zone of the East European Platform and the Southern. Chemosphere. 2021. Vol. 282. N 131106. DOI: 10.1016/j.chemosphere.2021.131106
- Rikhvanov L.P., Yazikov E.G., Baranovskaya N.V., Yankovich E.P. Patent N 2298212 RF. A method to determine areas of environmental pollution with uranium. Pub. 27.04.2007. Bul. N 12. (in Russian)
- Grigorev N.A. Distribution of chemical elements in the upper part of the continental crust. Ekaterinburg: UrO RAN, 2009, p. 382 (in Russian).
- Soktoev B.R., Rikhvanov L.P., Arynova Sh.Zh., Baranovskaya N.V. Natural radioactive elements (Th, U) in salt scales of natural fresh waters. Materialy V Mezhdunarodnoi konferentsii “Radioaktivnost' i radioaktivnye elementy v srede obitaniya cheloveka”, 13-16 sentyabrya 2016, Tomsk, Rossiya. OOO “STT”, 2016, p. 599-603 (in Russian).
