Assessment of collecting activity of physically sorbed reagents on the example of easily floatable coking coal sludge
Abstract
The article presents one of the new approaches to theoretical assessment of collecting ability of reagents. The efficiency of reagents-collectors with different chemical composition used for flotation of coking coals was studied. A comparative assessment of the flotation activity of kerosene, mineral oil, thermal gas oil, KETGOL and FLOTEK is given. The criteria of collecting activity of the above reagents-collectors for coal sludge flotation were specified. A correlation was established between the indicators of coal sludge flotation by the above reagents and their physical parameters. It is shown that the rate of spreading over water surface can characterize the flotation activity of reagents. Based on dependence of the collecting activity of a reagent on its rate of spreading along the “gas – liquid” interface and surface pressure, the main approaches to determining the structure and composition of molecules of an effective flotation collector can be determined. A new concept of the function performed by a physically sorbed collector in the elementary act of flotation and a criterion for the flotation activity of reagents used in coal sludge beneficiation are proposed. It is shown that the collector used in coal flotation, in addition to hydrophobizing the surface of the extracted particles, should reduce the induction time and remove the kinetic constraint on formation of a flotation aggregate.
Introduction
Currently, in Russia, coal sludge from coking coals is processed by the flotation beneficiation method at 25 coal preparation plants: in Kuzbass – 20 plants, in the Republic of Sakha-Yakutia – three, in the Khabarovsk Territory – one and in the Komi Republic – one.
Coal sludge is hydrophobic in nature; however, the reagents-collectors largely determine the technical and economic performance indicators of the process increasing its selectivity and speed. Physically sorbed collectors (PSC) are used for coal flotation. It is generally accepted that the main purpose of the PSC is an additional hydrophobization of coal surface.
At present, there is no sufficient clarity and generally accepted criteria linking the collecting activity of a reagent with its chemical and physical properties. To elucidate the action mechanism of nonpolar reagents, a numerous studies were carried out. It was supposed to establish a certain relationship between the yield of the flotation concentrate and the properties of a chemical compound, such as viscosity, volatility, solubility, molecular weight, dielectric constant, etc. The accumulated experimental material did not lead to unambiguous conclusions and a definite criterion of collecting activity. The purpose of the work is to determine the relationship between the surface-active properties of collecting reagents with respect to the “gas – liquid” interface and their collecting ability, to search for a criterion linking the flotation properties of hydrocarbons with their physicochemical characteristics.
Methodology
Nonpolar reagents are hydrocarbon liquids in molecules of which the dipole moment is low or absent. Most common among these collectors are diesel fuel (C12H24), dodecane (CH3(CH2)10CH3) and kerosene (mixture of hydrocarbons) [1]. A specific feature of molecules of nonpolar reagents is their inability to form a chemical bond with coal surface and a symmetrical structure of molecules. These features, in turn, determine the properties of nonpolar reagents: extremely low water solubility, low surface activity, and high hydrophobicity. A specific feature of nonionic heteropolar reagents is a significant surface activity in relation to the “gas – liquid” interface. They have a dipole moment, but at the same time, retain a limited solubility in water, for example, nonyl phenol (CH3-(CH2)8-C6H4-OH) which is a more efficient collector than dodecane [2].
Nonpolar compounds with an asymmetrical molecular structure and a dipole moment greater than zero are efficient. Nonpolar compounds with a symmetrical structure (benzene, heptane, hexane, octane, decane, dodecane, etc.) are not collectors. The authors [3, 4] come to the same conclusion. It has been ascertained that at the same flow rate, nonionic collectors with a dipole moment, for example, tetrahydrofurfuryl, are more efficient than, for example, dodecane with a symmetrical molecular structure. The surface of coal consists of hydrophobic areas and zones containing functional groups: carboxyl, carbonyl, phenolic and ether. Two interaction mechanisms of the reagent with mineral particles are proposed. The first mechanism of collector interaction with coal surface is due to a hydrogen bond between the polar groups of reagent and the oxidized functional groups on coal surface. It is similar to the mechanism of hydrophobization of ore minerals, but with a lower binding energy. The second mechanism arises as a result of water displacement from the hydrophobic surface of coal and formation of a bond between the reagent and the surface of coal. For the aliphatic chain of the reagent, this interaction is less pronounced than the interaction between the aromatic components of coal surface and benzene ring of the reagent. For example, as mentioned above, nonyl benzene is a more efficient collector than dodecane. A high collecting activity of nonyl benzene is due to a more intense interaction of benzene ring of the reagent with aromatic centers on coal surface. This interaction is due to the strong π-bonds between the aromatic components of coal surface and benzene ring of the reagent. The aliphatic hydrocarbon chain does not have such a bond with the surface of coal. At the same time and with the same consumption of collectors, the extraction of combustible matter by a heteropolar aliphatic reagent with a hydrophobic С3Н7 radical is much greater compared to consumption of the reagent containing a С6Н5 benzene ring. The same effect in collecting activity is manifested in collectors with hydrophobic radicals С7Н15 and С3Н6-С6Н5. The collecting activity of reagents with the functional group of tetrahydrofurfuryl increases with elongation of the hydrophobic fragment and reaches its maximum at a chain length corresponding to the oleate chain [3].
In paper [5], a comparative assessment of the collecting activity of hydrocarbons of the saturated (С6-С12), unsaturated series (С6-С14) as well as aromatic hydrocarbons (С6-С11) was given. Among the above types of hydrocarbons, aromatic compounds showed the maximum yield of concentrate. Among the aliphatic compounds, a higher concentrate yield was recorded for unsaturated hydrocarbons, their adsorption activity being determined by a double bond which is a combination of σ and π bonds. A comparison of energy parameters of the double and single bond shows that a double bond is much shorter and stronger than a single bond. However, the energy of a double bond is lower than that of two single bonds. Therefore, π-bonds are very mobile and easily polarized, which contributes to an increase in the interaction energy of alkenes and arenes with functional groups on the coal surface [5]. The strength of collector fixation on the coal surface is proposed as the main parameter of flotation activity of unsaturated hydrocarbons. It should be noted that during flotation of low-rank coals, hydroxy ethylated nonylphenol and hydroxy ethylated dodecyl ether showed similar results in terms of concentrate yield and ash content [6].
Studies have shown that at equal concentrations of reagents in water of 20·10–2 mol/m3, the adsorption of olefins on coal is 15 % higher than that of alkanes and arenes. Despite a lower sorption density, a higher yield of concentrate was recorded when using aromatic compounds [5]. The experiment showed the independence of concentrate yield from the sorption density of collector on coal.
At present, a unified view has formed on the action mechanism of nonpolar and heteropolar reagents based on a thermodynamic approach to an elementary act of flotation. According to this approach, the used reagents, nonpolar and heteropolar, are necessary for additional hydrophobization of the surface of coal sludges. Well floatable coal, not oxidized and not containing inorganic mineral inclusions, is well floated by nonpolar collectors. The surface of difficult-to-float coal contains hydrophilic functional groups: hydroxyl (–OH), carboxyl (–COOH), carbonyl (> C = O), and methoxyl (–O-CH3) [7-9]. For low-rank oxidized coal, the use of heteropolar collectors is mandatory [10-12]. Extraction of low-rank coal by carboxylic acids is more than twice as high as the extraction by saturated hydrocarbons [7]. In [13], it was ascertained that biodiesel fuel containing a large number of fatty acids is a more efficient collector in comparison with traditional diesel fuel. Oxidized diesel fuel increases the floatability of low-rank coal, since oxygen-containing functional groups on the coal surface, such as –OH, –COOH and –CO, can form a bond with oxygen-containing functional groups in oxidized diesel fuel [14-17]. In [18], crushed and oxidized coal was floated with dodecane and oleic acid. The advantage of using a combination of collectors-oleic acid and dodecane – for oxidized coal flotation has been experimentally proved. Coal sludge obtained by crushing immediately before the experiment can be floated with a high effect only by dodecane. An increase in the sorption density of the mixture obtained by adding cation-active n-octylamine to diesel fuel was recorded in [19]. Thus, the collectors containing functional groups are considered only in terms of increasing the fixation strength of a nonionic reagent and hydrophobicity of the mineral surface of low-rank coal. Kinetics of interaction of a coal particle with a gas bubble, the functions performed by reagents in this interaction are not considered by the authors. A correlation between floatability and contact angle is often not fulfilled, but it is it is always fulfilled with induction time.
Polar components of combined collectors KOBS, KETGOL containing С4-С10 alcohols are widely used. A high flotation activity of alcohols is explained by structuring of water aggregates, i.e., their hardening and expulsion of hydrophobic coal particles with an adsorbed layer of the same alcohols onto water surface [20, 21]. It is doubtful that the hydrophobic radical of alcohol oriented towards the surrounding water, can structure it; rather, the opposite phenomenon can be presumed. Foaming agents accelerate the flotation and have a positive effect on the process kinetics [22]. The authors attribute this phenomenon to the influence of foaming agents on the size of bubbles. As is known, low-molecular compounds, among them KOBS, KETGOL, have high kinetic characteristics in terms of diffusion and spreading over water surface. High flotation performance with the use of 2-ethyl hexanol is associated with its kinetic characteristics [23].
Nonpolar and heteropolar nonionic collectors fix on the coal surface as microdroplets [1, 24]. An increase in coal surface hydrophobization is achieved in two ways: by increasing the dispersiveness of emulsion with a corresponding growth of the number of microdroplets and by increasing the area covered by a microdroplet due to its spreading. Both problems are solved by reducing the surface tension of a traditional nonpolar collector (diesel fuel, dodecane, kerosene) by addition of surfactants. A decreasing surface tension leads to a growing contact angle of the collector droplet, i.e., its spreading over a solid surface. A slight increase in the coverage of coal surface with a flatter droplet of collector will increase its hydrophobicity. Kinetics of spreading of a nonpolar reagent droplet over coal surface was studied. The researchers presume that the main purpose of both traditional collectors and additional surface-active compounds is to increase hydrophobicity of coal surface. Thus, an increase in flotation performance with a decreasing surface tension of the collector can be accounted for by a better hydrophobization of the surface of coal particles.
It is stated in [25] that the presence in complex reagents of nonpolar compounds which have a high affinity for the aromatic mass of medium and highly metamorphosed coals and are also capable of fixation as droplets on coal surface, rather than as molecules, increases the efficiency of reagents. From this statement follows the need of a strong fixation of nonpolar compounds on coal surface, fixation of the above reagents in a droplet, and not in a molecular form. Fixation in a droplet form intensifies the use of reagents, and fixation according to the rule of equalizing the polarities of adjacent media increases the selectivity of extraction.
In [26], for a number of mixtures of foaming agents, an association was recorded between the surface activity in relation to the “gas – liquid” interface and floatability of various fractions of coal sludge. Three combinations of foaming agents were tested: alcohol + ketones, alcohol + compounds with an aldehyde group, and alcohol + polyglycol ether. Diesel fuel was used as the main collector. It has been ascertained that the mixture of foaming agents with a higher surface activity (alcohol + polyglycol ether) showed a greater yield of coal and a decrease in ash content. The authors do not disclose the reasons for the synergistic effect, in particular, from the use of a specific mixture. The effect of surface activity on flotation performance is not disclosed. It has also been experimentally proved that a higher concentrate yield and quality are achieved in the case of using a combined collector with a low surface tension in comparison with diesel fuel having a relatively high surface tension [24]. The possibility of a higher adsorption of the mixture of reagents on coal by reducing the interfacial tension between the collector and water is proved in [27].
In [7], the importance of choosing the length of the hydrocarbon radical of carboxylic acid is noted. With increasing number of atoms in a hydrocarbon fragment, the yield of a combustible matter first increases and then decreases. A similar dependence was also established in the flotation of ore minerals. This effect, according to the PSC operation mechanism, is explained by a change in removal rate of the liquid layer separating a mineral particle and a bubble at the time of the flotation aggregate formation [28]. An increasing concentrate yield with increasing length of the hydrocarbon chain to 8-12 carbon atoms is associated with a growing sorption by the collector mineral which has a higher activity in relation to the “gas – liquid” interface. At the time of the layer breakthrough, the surface pressure of the collector film forms causing its spreading. Subsequent reduction in concentrate yield is associated with a low spreading rate of the film of a long-chain (C > 12) collector as a result of the action of cohesion forces preventing spreading.
Paper [29] proves the influence of foaming agents on the wetting line displacement speed on solid surface. Formation kinetics of the contact perimeter of three media of different aggregate states in distilled water and solutions of n-octanol and α-terpineol was studied. An unexpected fact was established – at low concentrations of the foaming agent, the displacement speed of the contact perimeter increased in comparison with its movement speed in distilled water. An increase in concentration of foaming agent led to a slowdown in displacement of the contact perimeter.
A generally accepted approach which consists in hydrophobization of coal surface did not lead to an understanding of the formation mechanism of a flotation aggregate. The elaboration of criteria linking the collecting activity of a reagent with its chemical and physical properties cannot be based on a thermodynamic approach to the formation of a flotation aggregate. The value of free surface energy release characterized by the contact angle θ, is not a sufficient criterion for fixing a mineral particle since it does not take into account the kinetics of the process.
For searching the parameter that determines the flotation activity of reagents, the PSC mechanism was used. According to this mechanism, the purpose of the PSC is to remove the kinetic constraint to formation of a flotation aggregate. The kinetic constraint is a layer of liquid enclosed between the objects of interaction – a coal particle and a gas bubble. Natural hydrophobicity of coal, hydrodynamics of flows in the flotation machine cell allow the objects of interaction to approach at a distance at which a local breakthrough of the liquid layer is possible. The breakthrough is due to instability of the flotation system and reduction of its free surface energy according to (1). After a local breakthrough of the layer with formation of a meniscus connecting the particle with the bubble, the system “particle – bubble – liquid” comes to a stable state.
Energy at interfaces between the media of three aggregate states and the forming contact angle of the meniscus satisfy Young equation. However, the extent of the contact perimeter of three aggregate states is not sufficient for a stable fixation of a coal particle on a bubble. It is necessary to expand the contact perimeter of the media of three aggregate states to the length required for stable fixation of the value.
A physically fixed reagent disturbs the thermodynamic equilibrium as a result of spreading over the bubble surface, capturing and removing liquid from the layer [30]. Thinning of the layer leads to a decrease in the contact angle, its value does not correspond to the surface energies of the adjacent media, i.e., to violation of Young equation. Disturbance of thermodynamic equilibrium leads to a spontaneous expansion of the area of a “dry” spot on the surface of a coal particle – a return to a stable state. According to the PSC operation mechanism, the reagent spreading over water surface at a high speed accelerates the removal of liquid from the layer, reduces the induction time and has a high flotation activity. Due to this, the PSC operation mechanism was used to search for a criterion of flotation activity of a reagent for flotation of coal sludge.
Nonpolar and heteropolar collectors increase the size of floated particles, but the mechanism for increasing the size of recoverable particles remains debatable. It is assumed that the increase in particle size may be associated with a change in the size of bubbles, foam stability, and emulsifying properties. An increase in the size of the extracted particles is due to a local and short-term increase in surface tension at the “gas – liquid” interface adjacent to the mineral [31]. An increase in surface tension is recorded only at the time of the tearing force action. The force of capillary adhesion increases during detachment, and the tearing force caused by capillary pressure of gas in the bubble is retained. At the same time, the advancing contact angle is growing, which also increases the force of capillary adhesion.
Another point of view on the increasing size of floated particles as a result of the action of nonpolar reagents is presented in [32]. A positive role of nonpolar and heteropolar reagents in flotation of large fractions of coal is explained by a significant decrease in the inertial forces of detachment of coal particles from air bubbles as a result of amplitude damping and a decrease in the frequency of surface oscillations of bubbles. Damping of the amplitude of surface oscillations of a bubble and forced oscillations of particles on the bubble is explained by emergence of a gradient of surface concentration of collectors on bubbles changing their shape in a turbulent flow. Thus, the size of the extracted particles does not increase due to the strengthening “particle – bubble” contact, but as a result of a decrease in the inertial tearing forces. The performed calculations to determine the maximum size of the floated particles confirmed the adequacy of the proposed mechanism.
From the analysis of the considered papers, it follows:
- role of the reagent-collector in the elementary act of flotation consists in additional hydrophobization of coal;
- non-ionic nonpolar and heteropolar physically sorbed reagents have a low solubility;
- collectors fix on mineral surface as microdroplets. Decreasing surface tension of the nonpolar reagent with addition of surfactants intensifies the emulsification of the main collector and leads to flattening of the microdroplet on the coal surface;
- effective reagents have a certain surface activity in relation to the “gas – liquid” interface, a moderate foaming ability;
- foaming agents in small amounts increase the wetting line displacement speed on a solid surface at the time of the flotation contact formation and decrease it at elevated concentrations.
Chemical group composition and physicochemical properties of flotation reagents are presented in [33]. Determination of spreading rate of flotation reagents – kerosene, mineral oil, thermal gas oil, KETGOL and FLOTEK over water surface by high-speed shooting technique was made on the unit presented in [33].
Flotation experiments were performed on coal sludge of technological Zh rank from the Elginsky coal field (Republic of Sakha), the Pechora coal basin (Komi Republic), and the Kuznetsk coal basin (Kuzbass). Petrographic studies of coal sludges were performed on the automated complex SIAMS 620 (Table 1).
Table 1
Petrographic studies and elemental analysis of combustible matter
|
Name of sample/coal rank |
Ash content of initial sludge, % |
Reflectivity Ro, % |
Petrophysical composition, % |
Elemental analysis |
|||||||
|
Vitrinite Vt |
Leptynite L |
Semivitrinite Sv |
Fusinite F |
Mineral impurities Me |
Carbon C |
Hydrogen Н |
Nitrogen N |
Oxygen O |
|||
|
N 1/Zh (Sakha Republic) |
15.50 |
0.83 |
87 |
– |
2 |
10 |
Present |
84.3 |
4.8 |
1.8 |
2.62 |
|
N 2/Zh (Komi Republic) |
26.40 |
0.78 |
89 |
– |
– |
8 |
Present |
82.4 |
4.6 |
2.5 |
4.3 |
|
N 3/Zh (Kuzbass) |
21.70 |
0.80 |
94 |
1 |
– |
4 |
Present |
85.1 |
5.8 |
2.9 |
5.6 |
Granulometric composition of coal sludges is determined pursuant to GOST 2093-82 and is given in Table 2. The predominant classes are 0.2-0.5 and 0.1-0.2 mm. Coal particles of this size, as a rule, have a good floatability [34]. Fractional composition of the sludge from the above fields is given in Table 3.
In accordance with GOST 10100-84, the presented coal sludge of Zh rank refers to the easy washability category.
The main part of the research was focused on flotation experiments in accordance with GOST 33656-2015 under laboratory conditions on FMP-L1 flotation machine. Flotation was performed with the following reagents: kerosene, mineral oil, thermal gas oil, KETGOL and FLOTEK. Sludges of easily floatable coal served as the material for flotation. During the experiments on flotation, constant conditions were maintained: size of floated coal sludge 0-0.5 mm, S:L ratio = 1:3, water temperature 20 °С, stirrer rotation speed 1,500 rpm, consumption of reagents 500 g/t, contact time with reagents 2 min, skimming time 5 min.
Table 2
Grain size of sludge
|
Ranks, mm |
Sample N 1 |
Sample N 2 |
Sample N 3 |
|||
|
Yield, % |
Ash content, % |
Yield, % |
Ash content, % |
Yield, % |
Ash content, % |
|
|
0.2-0.5 |
60.41 |
11.95 |
42.67 |
23.97 |
42.06 |
22.85 |
|
0.1-0.2 |
14.74 |
16.40 |
28.70 |
25.77 |
29.00 |
21.10 |
|
0.05-0.1 |
12.63 |
22.15 |
9.63 |
27.89 |
12.15 |
19.96 |
|
0-0.05 |
12.22 |
25.10 |
19.00 |
32.06 |
16.79 |
21.10 |
|
TOTAL |
100.0 |
15.50 |
100.00 |
26.40 |
100.00 |
21.70 |
Table 3
Fractional composition of sludge
|
Fraction density, kg/m3 |
Sample N 1 |
Sample N 2 |
Sample N 3 |
|||
|
Yield, % |
Ash content, % |
Yield, % |
Ash content, % |
Yield, % |
Ash content, % |
|
|
Less than 1,300 |
32.20 |
4.49 |
34.70 |
2.90 |
54.95 |
5.90 |
|
1,300-1,400 |
42.20 |
7.30 |
25.33 |
6.30 |
18.93 |
11.90 |
|
1,400-1,500 |
11.30 |
15.90 |
8.47 |
12.70 |
4.93 |
20.10 |
|
1,500-1,600 |
2.00 |
26.70 |
1.50 |
19.80 |
1.63 |
27.30 |
|
1,600-1,800 |
2.20 |
41.80 |
1.88 |
30.60 |
1.80 |
39.20 |
|
Over 1,800 |
10.10 |
76.50 |
28.12 |
77.70 |
17.76 |
79.20 |
|
TOTAL |
100.00 |
15.50 |
100.00 |
26.40 |
100.00 |
21.70 |
|
Т = 4.67 % |
Т = 4.7 % |
Т = 4.17 % |
||||
Spreading rate of the studied reagents over water surface depending on time was determined earlier [33]. Spreading rate of collectors was determined on the surface of tap water; pH and temperature were chosen equal to the temperature and pH of the flotation pulp. Initial spreading rate was: kerosene – 9; mineral oil – 13; thermal gas oil – 18; KETGOL – 21.5 and FLOTEK – 26 cm/s.
Flotation experiments were conducted to assess the collecting activity of the studied reagents and compare it with the rate of spreading along the “gas – liquid” interface. The following technological indicators of flotation were determined: flotation concentrate yield γc; ash content of flotation concentrate Acd extraction of combustible (useful) matter to concentrate Ec.m; ash content of flotation rejects (tailings) Atd. Calculations were performed using the formulas:
where m1 is the mass of ignited sampling boat, g; m2 – mass of sampling boat with coal subsample, g; m3 – mass of sampling boat with ignition rejects, g;
The results of laboratory experiments on flotation of easily floatable coal sludge using reagents of different groups are presented in Table 4.
A comparison of combustible matter extraction into a concentrate and spreading rate of flotation reagents over water surface is shown in the Figure.
Table 4
Flotation results of easily floatable Zh rank coal sludge depending on the efficiency of reagents, %
|
Number of experiment |
Name of collector |
Collector |
Initial ash content |
Concentrate |
Tailings |
Еc.m, % |
||
|
Yield, % |
Ash content, % |
Yield, % |
Ash content, % |
|||||
|
Sample N 1, Sakha Republic |
||||||||
|
1 |
Kerosyne |
500.00 |
15.50 |
62.56 |
12.89 |
37.44 |
19.86 |
64.49 |
|
2 |
Mineral oil |
500.00 |
15.50 |
65.89 |
12.54 |
34.11 |
21.22 |
68.20 |
|
3 |
Thermal gas oil |
500.00 |
15.50 |
80.76 |
11.25 |
19.24 |
33.34 |
84.82 |
|
4 |
KETGOL |
500.00 |
15.50 |
82.34 |
10.11 |
17.66 |
40.63 |
87.59 |
|
5 |
FLOTEK |
500.00 |
15.50 |
86.22 |
8.55 |
13.78 |
58.99 |
93.31 |
|
Sample N 2, Komi Republic |
||||||||
|
1 |
Kerosyne |
500.00 |
26.40 |
55.70 |
16.10 |
44.30 |
39.35 |
63.49 |
|
2 |
Mineral oil |
500.00 |
26.40 |
57.77 |
14.66 |
42.23 |
42.46 |
66.98 |
|
3 |
Thermal gas oil |
500.00 |
26.40 |
62.17 |
10.23 |
37.83 |
52.97 |
75.83 |
|
4 |
KETGOL |
500.00 |
26.40 |
63.10 |
9.67 |
36.90 |
55.01 |
77.44 |
|
5 |
FLOTEK |
500.00 |
26.40 |
68.55 |
8.31 |
31.45 |
65.83 |
85.40 |
|
Sample N 3, Kuzbass |
||||||||
|
1 |
Kerosyne |
500.00 |
21.70 |
61.11 |
14.60 |
38.89 |
32.86 |
66.65 |
|
2 |
Mineral oil |
500.00 |
21.70 |
63.98 |
12.10 |
36.02 |
38.75 |
71.82 |
|
3 |
Thermal gas oil |
500.00 |
21.70 |
67.88 |
11.89 |
32.12 |
42.43 |
76.38 |
|
4 |
KETGOL |
500.00 |
21.70 |
70.78 |
11.23 |
29.22 |
47.06 |
80.24 |
|
5 |
FLOTEK |
500.00 |
21.70 |
76.00 |
9.70 |
24.00 |
59.70 |
87.65 |
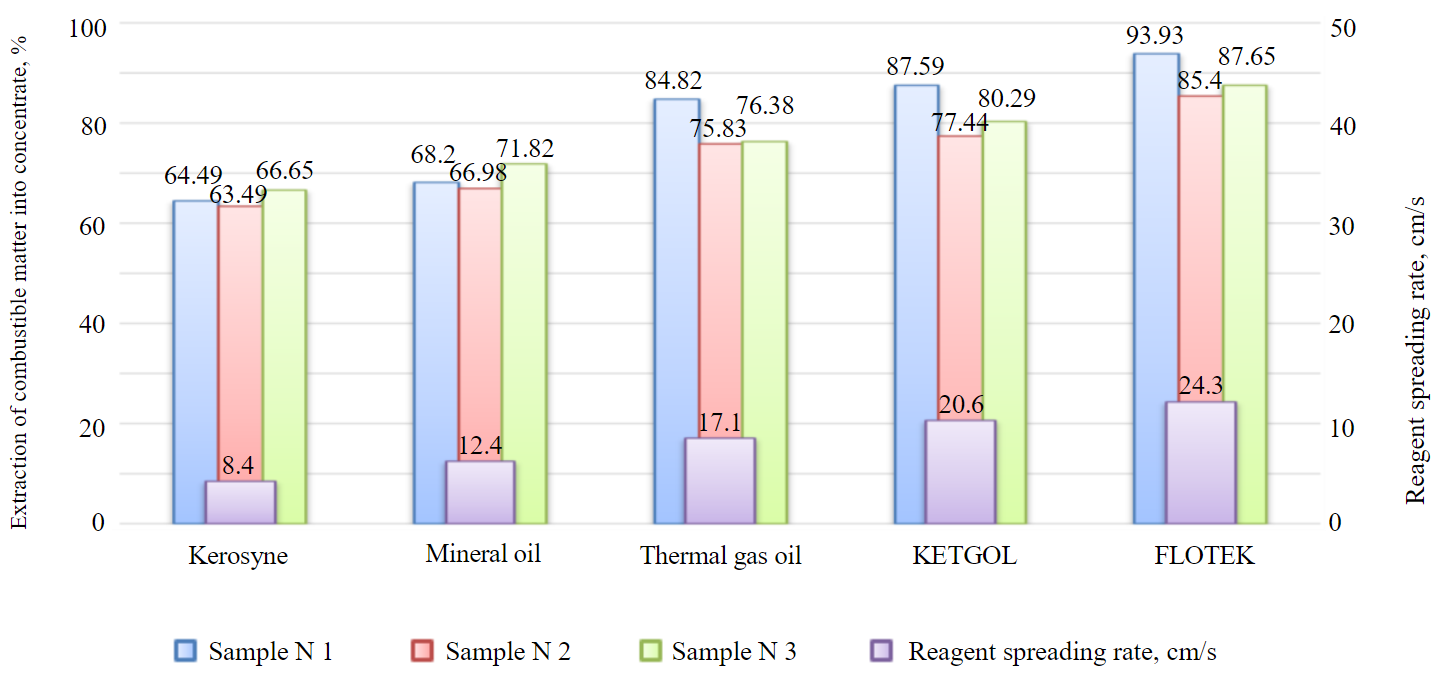
Extraction of combustible matter into concentrate and spreading rate of flotation reagents
Discussion
From Table 4 it follows that the extraction value depends on the coal field: in case of flotation with FLOTEK reagent for easily floatable Zh rank coal sludge, sample N 2, it was 85.40; sample N 3 – 87.65 %; sample N 1 – 93.31 %. Ranking of reagents-collectors based on flotation activity does not change with a coal field. According to the operation mechanism of physically sorbed collectors, the extraction is determined by their activity in relation to the “gas – liquid” interface. The initial spreading rate of collectors over water increases in the following series: kerosene – mineral oil – thermal gas oil – KETGOL – FLOTEK. Collecting activity of these reagents is increasing in the same sequence. Experimental results point to a correlation between flotation efficiency of the collector and its surface activity and spreading rate over water. Collectors with surface activity, low solubility, moderate foaming capacity meet the requirements that are necessary for spreading over water and removing the kinetic constraint on formation of the flotation aggregate. Collectors have a dipole moment which is necessary for interaction with water molecules and spreading along the “gas – liquid” interface.
The specified advantages of heteropolar collectors consist in their ability to interact with mole-cules of water and spread over its surface. Assumptions about their hydrophobic function are not convincing since they fix as microdroplets according to the rule of equalizing the polarities of adja-cent media. Diphilic molecules of a heteropolar collector contained in a microdroplet have their nonpolar part facing the coal surface. Polar part of molecules is facing water molecules. In low-rank coal, molecules of ionogenic collectors in a microdroplet can form a bond with its oxidized surface. However, on the surface of a microdroplet adjoining water, functional groups of the collector molecules will also be oriented in its direction. Fragmentary fixation generates a chemical heterogeneity of the surface and slows down the movement of the wetting line along the coal surface.
A relatively high flotation activity of С8-С12 hydrocarbons [5] can be accounted for by two factors. First, when the hydrocarbon fragment of the collector contains less than 6-8 carbon atoms, their sorption activity decreases. After breakthrough of the layer, the concentration gradient of molecules on the mineral and the bubble surface is insufficient for the development of a surface flow of collector. Second, the removal rate of the liquid layer decreases with increasing length of the hydrocarbon fragment over 12 atoms as a result of a decreasing spreading rate caused by cohesion of the collector molecules [28]. In case of 8-12 carbon atoms, the reagent is adequately sorbed, and the collector film has the maximum spreading rate and the maximum rate of liquid removal from the layer.
Nonyl benzene is a more efficient collector than dodecane due to an asymmetric structure of its molecules and a certain dipole moment. These properties allow the molecules to interact with dipoles of water and spread over its surface. Assumption of a higher flotation activity of nonyl benzene in comparison with aliphatic reagents is insufficiently substantiated. The simplest compounds of the aromatic series with a symmetrical molecular structure, for example, benzene, are flotation-inert [3]. Such reagents allow coal flotation only at a very high cost. Low flotation properties of benzene are explained by the same electron density of carbon atoms in unsubstituted and symmetrical aromatic molecules. When various substituents enter the aromatic ring, electron density is redistributed depending on the type of substituents, their position and number in the molecule. The authors [3] presume that an irregular distribution of electron density will be more consistent with the distribution of active centers on the inhomogeneous surface of coal. There is no evidence that the distribution of electron density of the collector corresponds to the distribution of active centers on the surface of coal. The PSC mechanism of operation offers another concept for the formation of the flotation complex “coal particle – gas bubble”. Tetrahydrofurfuryl is more efficient than, for example, dodecane due to appearance of a dipole moment, the ability to spread over water surface and remove the kinetic constraint to formation of a flotation aggregate. Therefore, the assumption that the reagent acquires a dipole moment, of the ability to interact with water molecules and remove liquid from the layer between the objects of interaction seems more convincing. The above mechanism of reducing the time of particle fixation by heterogeneous collectors is associated with a decrease in induction time. Increase of liquid removal rate from the layer enclosed between the objects of interaction reduces the time of coal particle fixation on the bubble and, as a consequence, flotation time as a whole. The established correlation between the flotation activity of the collector and the rate of its spreading over water confirms this conclusion.
A high collecting activity of tetrahydrofurfuryl oleate (C4H7O-CH2-OOC-C7H14-CH = CH-C8H17) is explained by a decrease in lateral interaction of hydrocarbon radicals in comparison with other radicals of this collector. Therefore, for reducing the solubility and achieving a high spreading rate, it was necessary to increase the length of the hydrocarbon chain and introduce a double bond into it. A similar effect will be observed when using branched hydrocarbons as a collector.
An extreme behavior of the wetting line displacement speed [29] depending on concentration of the foaming agent is due to a difference in its adsorption on a solid surface and a bubble. At low concentrations, its sorption on a rising bubble is lower than on a solid surface that was in liquid for a long time. Increasing concentration of the foaming agent levels this difference, which will lead to a decrease in its surface flow and slow down the movement of the wetting line.
Composition and structure of molecules of an efficient collector fully correspond to the properties of the reagent necessary to perform the function of removing liquid from the layer separating a coal particle and a gas bubble. Properties of the collector also correspond to a change in composition and structure of the surfactant molecule, which is highly efficient in lowering the surface energy at “gas – liquid” interface [35]. Driving force of spreading is surface pressure π = σGL – σOil, where σGL is surface tension of water; σOil, surface tension of water after the collector spreads. Surface pressure can be used as a criterion of flotation activity of a physically sorbed collector. The proposed criterion is subject to constraints. For collectors with a developed hydrocarbon radical significant cohesive forces will prevent the collector from spreading and increase the induction time. For short-chain collectors, high solubility and low adsorption will reduce the concentration gradient of the collector at the time of a breakthrough of the liquid layer, which will also lead to an increase in the induction time [36].
Conclusions
A new concept of the function performed by the physically sorbed collector in the elementary act of flotation is proposed. This reagent, in addition to hydrophobizing the surface of extracted particles, reduces the induction time and removes the kinetic constraint to formation of
a flotation aggregate.
A correlation has been established between the spreading rate of nonpolar and heteropolar reagents over water surface and extraction of combustible matter into a concentrate. It has been proved experimentally that when selecting reagents for the flotation of easily floatable coal sludges it is necessary to use compounds that are well sorbed by coal and have a high spreading rate over water surface.
Based on dependence of collecting activity of reagent on its spreading rate along the “gas – liquid” interface and surface pressure, the main approaches to determining the structure and composition of the molecules of an efficient flotation collector can be determined.
References
- Kadagala M. R., Nikkam S., Tripathy S.K. A review on flotation of coal using mixed reagent systems. Minerals Engineering. 2021. Vol. 173. N 107217. DOI: 10.1016/j.mineng.2021.107217
- Jia R., Harris G. H., Fuerstenau D.W. An improved class of universal collectors for the flotation of oxidized and /or low-rank coal. International Journal of Mineral Processing. 2000. Vol. 58. Iss. 1-4, p. 99-118. DOI: 10.1016/S0301-7516(99)00024-1
- Jia R., Harris G. H., Fuerstenau D.W. Chemical Reagents for Enhanced Coal Flotation. Coal Preparation. 2002. Vol. 22. Iss. 3, p. 123-149. DOI: 10.1080/07349340213847
- Zhenyong Miao, Yaowen Xing, Xiahui Gui et al. Anthracite Coal Flotation Using Dodecane and Nonyl Benzene. International Journal of Coal Preparation and Utilization. 2018. Vol. 38. Iss. 8, p. 393-401. DOI: 10.1080/19392699.2016.1277209
- Osina N.Yu., Gorokhov A.V., Lakhtin S.V. Study of the influence of chemical group composition of collector reagents on the efficiency of hard coal flotation. Mining informatsional and analytical bulletin. 2006. N 2, p. 393-396 (in Russian).
- Bao Li, Jianying Guo, Shengyu Liuet al. Molecular insight into the mechanism of benzene ring in nonionic surfactants on low-rank coal floatability. Journal of Molecular Liquids. 2020. Vol. 302. N 112563. DOI: 10.1016/j.molliq.2020.112563
- Quanzhi Tian, Yi Zhang, Guosheng Li, Yongtian Wang. Application of Carboxylic Acid in Low-Rank Coal Flotation. International Journal of Coal Preparation and Utilization. 2019. Vol. 39. Iss. 1, p. 44-53. DOI: 10.1080/19392699.2017.1297299
- Ni C., Xie G., Li Z. et al. Flotation of long flame coal pretreated by polyoxyethylene sorbitan monostearate. Physicochemical Problem of Mineral Processing. 2016. Vol. 52. Iss. 1, p. 317-327. DOI: 10.5277/ppmp160127
- Zhang H., Liu Q. Lignite cleaning in NaCl solutions by a reverse flotation technology. Physicochemical Problem of Mineral Processing. 2015. Vol. 51, p. 695-706. DOI: 10.5277/ppmp150227
- Yangchao Xia, Zili Yang, Rui Zhang et al. Performance of used lubricating oil as flotation collector for the recovery of clean low-rank coal. Fuel. 2019. Vol. 239, p. 717-725. DOI: 10.1016/j.fuel.2018.11.086
- Dey S. Enhancement in hydrophobicity of low rank coal by surfactants – a critical overview. Fuel Processing Technology. 2012. Vol. 94. Iss. 1, p. 151-158. DOI: 10.1016/j.fuproc.2011.10.021
- Jena M.S., Biswal S.K., Rudramuniyappa M.V. Study on flotation characteristics of oxidized Indian high ash sub-bituminous coal. International Journal of Mineral Processing. 2008. Vol. 87. Iss. 1-2, p. 42-50. DOI: 10.1016/j.minpro.2008.01.004
- Wencheng Xia. Bio-diesel as renewable collector for coal flotation in the future. Energy Sources. Part A. Vol. 38. Iss. 13, p. 1938-1943. DOI: 10.1080/15567036.2015.1020461
- Wencheng Xia, Jianguo Yang. Enhancement in flotation of oxidized coal by oxidized diesel oil and grinding pretreatment. International Journal of Coal Preparation and Utilization. 2013. Vol. 33. Iss. 6, p. 257-265. DOI: 10.1080/19392699.2013.816300
- Baofeng Wen, Wencheng Xia, Sokolovic J.M. Recent advances in effective collectors for enhancing the flotation of low rank/oxidized coals. Powder Technology. 2017. Vol. 319, p. 1-11. DOI: 10.1016/j.powtec.2017.06.030
- Ziyong Chang, Xumeng Chen, Yongjun Peng. The interaction between diesel and surfactant Triton X-100 and their adsorption on coal surfaces with different degrees of oxidation. Powder Technology. 2019. Vol. 342, p. 840-847. DOI: 10.1016/j.powtec.2018.10.047
- Yaowen Xing, Xiahui Gui, Yijun Cao et al. Effect of compound collector and blending frother on froth stability and flotation performance of oxidized coal. Powder Technology. 2017. Vol. 305, p. 166-173. DOI: 10.1016/j.powtec.2016.10.003
- Yaowen Xing, Chenwei Li, Xiahui Gui, Yijun Cao. Interaction Forces between Paraffin / Stearic Acid and Fresh/Oxidized Coal Particles Measured by Atomic Force Microscopy. Energy & Fuels. 2017. Vol. 31. Iss. 3, p. 3305-3312. DOI: 10.1021/acs.energyfuels.6b02856
- Xu M., Xing Y., Li M. et al. Oxidized coal flotation enhanced by adding noctylamine. Energy Sources. Part A: Recovery, Utilization, and Environmental Effects. 2018. Vol. 40. Iss. 20, p. 2394-2399. DOI: 10.1080/15567036.2018.1495787
- Chuprova L.V. Studying of the Mechanism of Effect of Reagents at Floatation Beneficiation of Coal Slimes. International Journal of Applied and Fundamental Research. 2016. N 11, p. 939-942 (in Russian).
- Miroshnikov A.M., Ivanov G. V., Azarova T. I., Sukharevskaya G. K. On the action mechanisms of glycol alcohols and ethers in coal beneficiation. Izvestiya vysshikh uchebnykh zavedenii. Gornyi zhurnal. 2010. N 3, p. 93-97 (in Russian).
- Yinfei Liao, Yijun Cao, Shaomeng Huang et al. Water-carrying properties of flotation frothers and its effect on fine coal flotation. International Journal of Coal Preparation and Utilization. 2015. Vol. 3. Iss. 2, p. 88-98. DOI: 10.1080/19392699.2014.976705
- Jinzhou Qu, Xiuxiang Tao, Huan He et al. Synergistic Effect of Surfactants and a Collector on the Flotation of a Low-Rank Coal. International Journal of Coal Preparation and Utilization. 2015. Vol. 35. Iss. 1, p. 14-24. DOI: 10.1080/19392699.2014.904295
- Ding L.P. Effect of Collector Interfacial Tension on Coal Flotation of Different Particle Sizes. Industrial & Engineering Chemistry Research. 2010. Vol. 49. Iss. 8, p. 3769-3775. DOI: 10.1021/ie901813j
- Kubak D.A., Petukhov V. N., Semenov D. G. Study of the influence of chemical group composition of complex reagents on the efficiency of coal flotation. Vestnik MGTU im. G.I.Nosova. 2013. N 4, p. 5-10 (in Russian).
- Ashiwani Kumar Gupta, Banerjee P.K., Arun Mishra. Influence of chemical parameters on selectivity and recovery of fine coal through flotation. International Journal of Mineral Processing. 2009. Vol. 92. Iss. 1-2, p. 1-6. DOI: 10.1016/j.minpro.2009.02.001
- Rui Zhang, Yangchao Xia, Fangyu Guo et al. Effect of microemulsion on low-rank coal flotation by mixing DTAB and diesel oil. Fuel. 2020. Vol. 260. N 11632. DOI: 10.1016/j.fuel.2019.116321
- Kondratev S.A., Moshkin N.P. Assessment of collecting activity of a flotation reagent. Fiziko-tekhnicheskie problemy razrabotki poleznykh iskopaemykh. 2015. N 1, p. 137-144 (in Russian).
- Kosior D., Zawala J., Malysa K. When and how α-terpineol and n-octanol can inhibit the bubble attachment to hydrophobic surfaces. Physicochemical Problems of Mineral Processing. 2011. Vol. 47, p. 169-182.
- Kondratev S.A. Collectability and Selectivity of Flotation Agent. Fiziko-texhnicheskiye problemy razrabbotki poleznykh iskopaemykh. 2021. N 3, p. 133-147 (in Russian). DOI: 10.15372/FTPRPI20210313
- Melik-Gaikazyan V.I. Interphase interactions. Physicochemical fundamentals of flotation theory. Moscow: Nauka, 1983, p. 22-48 (in Russian).
- Kondratev S.A. Reagents-collectors in an elementary flotation act. Novosibirsk: Sibirskoe otdelenie Rossiiskoi akademii nauk, 2012, p. 240 (in Russian).
- Khamzina T.A., Kondratev S.A. Activity of Different Chemistry Agents in Flotation of Difficult Slack Coal. Fiziko-texhnicheskiye problemy razrabbotki poleznykh iskopaemykh. 2021. N 4, p. 121-131 (in Russian). DOI: 10.15372/FTPRPI20210412
- Barsukova A.Ya., Vlasova N.S., Kosorukova T.V. On flotation of coal particles of different size: Problemy obogashcheniya i briketirovaniya uglya. Moscow: Nedra, 1987, p. 60-66 (in Russian).
- Rosen M.J. Reduction of Surface and In-terfacial Tension by Surfactants. Surfactants and interfacial phenomena. New York: Hoboken, John Wiley & Sons, Inc., 2004, p. 208-242.
- O'Brien R.N., Feher A.I., Leja J. Spreading of Monolayers at the Air-Water Interface II. Spreading Speeds for Alcohols, Acids, Esters, Sulphonates, Amines, Quaternary Ammonium Ions, and Some Binary Mixtures. Journal of Colloid and Interface Science. 1976. Vol. 56. Iss. 3, p. 474-482. DOI: 10.1016/0021-9797(76)90113-2
