Natural carbon matrices based on brown coal, humic acids and humine extracted from it for purification of aqueous solutions from low molecular weight organic impurities
- 1 — Ph.D., Dr.Sci. Professor Kyrgyz National University named after Zhusup Balasagyn ▪ Orcid
- 2 — Applicant, Engineer Kyrgyz National University named after Zhusup Balasagyn ▪ Orcid
- 3 — Ph.D. Associate Professor Kyrgyz National University named after Zhusup Balasagyn ▪ Orcid
- 4 — Ph.D. Head of Laboratory (Saint Petersburg Research Center for Environmental Safety of the RAS ▪ Orcid
- 5 — Ph.D. Senior Researcher Saint Petersburg Research Center for Environmental Safety of the RAS ▪ Orcid
Abstract
Heterogeneous systems including natural carbon matrices in the solid phase and aqueous solutions of low molecular weight organic compounds with positive and negative variations from ideality in the liquid phase are considered. The technical characterization of the considered supramolecular ensembles on the basis of brown coal of the Kara-Keche deposit (Kyrgyzstan), humic acids and humine extracted from it is given. Functional analysis of the samples was carried out using FTIR spectroscopy. The morphology of the surface of the considered carbon matrices has been investigated, in different points of which the local microelement composition has been established. An X-ray phase analysis of Kara-Keche brown coal and humic acids and humine extracted from it was carried out. The isothermal adsorption of bipolar molecules of glycine and urea, neutral D-glucose from aqueous solutions on solid carbon sorbents has been studied. An assumption has been made about the adsorption of low molecular weight organic compounds from aqueous solutions on humine and Kara-Keche coal in irregularities and pores of the carbon matrix of sorbents, for humic acids – on surface reaction centers. Due to its developed pore structure and resistance to acids and alkalis, humine from Kara-Keche coal is recommended for the purification of industrial wastewater from low molecular weight organic ecotoxicants.
Funding
Research supported by government research theme № 122041100143-5, code FFZF-2022-0014
Introduction
Purification of wastewater from industrial plants remains one of the main environmental problems, the solution of which will help combat the threat of pollution of natural objects. A material of natural origin – brown coal – is widely used as an absorber of ecotoxicants. The main advantages of this sorbent are high sorption capacity, environmental friendliness and a wide range of applications. It has a heterogeneous structure, which consists of a huge number of different functional groups [1-3]. Brown coal is a potential source of humic substances (HS), which have a wide spectrum of effects. Their content in brown coals ranges from 20 to 90 %. The share of the mineral part is about 52 % [4]. Brown coal from the point of view of solid-state chemistry is an interesting object for mechanochemical oxidation aimed at increasing the content of humic acids and chemical modification of their structure. The most studied is the solid-phase treatment of brown coal with alkalis, leading to an increase in the extractability of humic acids [5].
A large number of reviews are devoted to humic substances [6-8], which have a variety of specific properties that ensure their wide practical use. The works [9-11] note the ability of humic acids (HA) to effectively bind metal cations. The processes of binding of copper, nickel, cadmium ions, ferricenium cations and methylene blue from aqueous solutions on humine (residual coal) and humic acid of Kara-Keche brown coal have been studied in detail [12,13].
The interaction of organic compounds with HS allows their use in the production of microfertilizers, feed and food additives, and as enterosorbents for water purification and detoxification of land from petroleum products [14-16]. Research continues on the development of technical specifications for the use of products based on recycled organic waste as sorption material for the treatment of domestic, industrial and technological wastewater from organic and inorganic components [17,18]. The interaction of HS with mineral particles of the soil with the formation of organomineral complexes determines their use as structure formers and soil ameliorants [19]. The prospects of using humic preparations for restoration of disturbed areas are investigated by assessing the relationship between their biological activity and structural-group composition [20-22].
Various thermodynamic and kinetic models are used to describe the sorption process involving HS [23-25]. Using these models, as well as ideas about the supramolecular nature of HS, it is possible to understand the mechanism of the process, select the most effective sorbents and optimal conditions for sorption [26-28].
Insufficient attention is paid to adsorption of low molecular weight organic compounds from aqueous solutions on humic acids and especially on humine extracted from brown coals [29-31]. There are few publications in the scientific literature on functional and local trace element compositions, surface morphology of brown coal-based carbon sorbents, humic acids and humine extracted from them [32-34].
The limited information causes the actuality of studies of surface properties in heterogeneous systems including water-organic solutions with positive and negative variations from ideality, which are contained in wastewater of chemical, pharmaceutical, electrical engineering. The treatment of wastewater from low molecular weight organic compounds is an important environmental protection goal. At the same time, humin (residual coal) as a spent carbon sorbent can be utilized by combustion in power plants as a refined fuel.
The aim of the work is the physicochemical characterization of brown coal of Kara-Keche deposit (Kyrgyzstan), humic acids and humine extracted from it, determination of their classification features, study of isothermal adsorption of low molecular weight organic compounds from aqueous solutions on the obtained natural carbon matrices.
Methods
One of the ways to extract humic acids from brown coals is alkaline extraction [35]. At the first stage of humic acid and humine extraction, decalcification of the sample was carried out to free the coal and organic compounds contained in it from calcium ions, which form difficultly soluble humates with humic acids. For this purpose, a sample of coal was filled with 0.005 n solution of Н2SO4 at the rate of 5-6 liters per 1 kg of coal, stirred for 15 min on a rotator and left for 24 h. After settling, the acid solution was drained and discarded along with the undecomposed plant materials that floated to the surface. Upon exposure to mineral acids, aluminium and iron partially pass into solution, which also contributes to the release of humic substances.
After decalcification, the coal was washed with distilled water and humic acids were extracted with 0,1 n NaOH solution at the rate of 6-7 liters per 1 kg of coal. Stirred for 15-20 min on a rotator, then the suspension was allowed to stand for 24 h followed by separation of the solid phase by centrifugation at 4000 rpm. The mother liquor was sodium humates, the undissolved precipitate was the non-hydrolysable part of the charcoal (humine).
Humine was placed in a 10-litre container, poured with distilled water, mixed thoroughly and allowed to stand for two days. After settling, the upper clear layer was drained (by decantation) and the lower layer was centrifuged on an OS-6M centrifuge at 4000 rpm for 15 min.
The resulting humic precipitate was rewashed six more times to remove residual amounts of humic acids and small dispersed sols, which give colour to the solutions. After final centrifugation, the precipitate was placed on glass and dried at room temperature. It was then dried in a desiccator for one hour at 90-100 °C, crushed and ground in a mortar to a powdery state. The obtained humic sample from Kara-Keche coal was sieved on sieves with 0.25 mm mesh diameter (60 mesh). Humine was stored in tightly corked bouquets for further research.
The solution after coal leaching usually contains some amount of mineral impurities in the form of a fine suspension, from which were freed before the humic acids were precipitated. For this purpose, the alkaline solution was centrifuged at 4000 rpm. To precipitate humic substances a small amount of concentrated H2SO4 at the amount of 2.0-2.5 ml per 1 liter of alkaline solution was added to the obtained solution (until the first signs of coagulation). The loose precipitate of humic acids was centrifuged to separate it from the solution, transferred to a filter and washed several times with distilled water to neutral pH value. The crude preparation of humic acids contains a certain amount of himatomelanic acids, for the separation of which the precipitate was washed with ethyl alcohol, and the himatomelanic acids passed into solution.
The wet humic acid precipitate was air-dried for one or two days and then pe-recrystallised. For purification from small amounts of impurities humic acids were again dissolved in a small amount of 0.1 n NaOH solution at the rate of 30 ml of alkali per 10 g of humic acids, stirred for 15 min and stood for 24 hours. The humic acids were further extracted from the alkaline solution. The purified humic acid preparation was then air dried and ground to a powder-like state and sieved on sieves with a mesh diameter of 0.25 mm. The obtained preparation was stored in the desiccator.
Discussion of results
Ash content Aa and moisture Wa of the analysed sample of Kara-Keche coal, humic acids and humine extracted from it were obtained according to the methods of [36] (Table 1).
Table 1
Moisture and ash content of Kara-Keche brown coal, humic acids and humine extracted from it, elemental analysis in count to ash-free, anhydrous mass of samples
|
Object of the study |
Wa, % |
Aa, % |
Сh, % |
Sh, % |
Нh, % |
Nh, % |
Оh, % |
Н/С |
О/С |
|
Brown coal (CK) |
2,54 |
22,90 |
72,33 |
1,21 |
4,72 |
0,98 |
20,76 |
0,78 |
0,22 |
|
Humic acids from coal (HA) |
3,63 |
10,70 |
70,90 |
1,22 |
3,56 |
0,62 |
23,70 |
0,60 |
0,25 |
|
Humine from coal (HU), washed |
7,35 |
20,52 |
78,75 |
1,29 |
3,94 |
0,00 |
16,02 |
0,60 |
0,15 |
The carbon content of humine increases and the oxygen content decreases markedly compared to humic acids and coal itself (Table 1). Humic acid structures were estimated from the hydrogen-to-carbon and oxygen-to-carbon content ratios using the Van-Crevelen graph-static analysis method. According to classification criteria, the humic acids extracted from Kara-Keche coal belong to the class of humic acids from brown coals and the black soil.
Functional analysis of Kara-Keche coal, humic acids and humine extracted from it by FT-IR spectroscopy. Near-infrared spectroscopy is widely used in both qualitative and quantitative analyses [37, 38] and is also used in the study of brown coals.
Figure 1 shows the IR spectra of all investigated samples recorded on Varian FT-IR spectrometer in tablets with KBr in the ratio 1:300, frequency range 4000-450 cm–1, as well as the maxima of characteristic absorption bands of the most important atomic groups of the investigated substances.
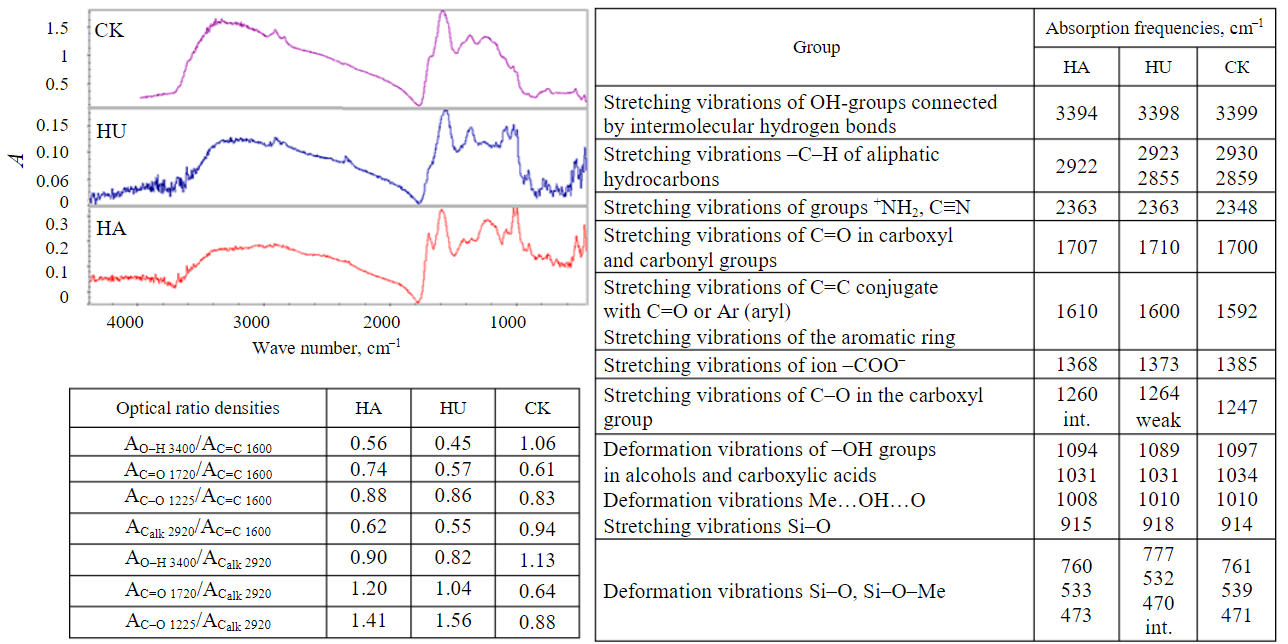
Fig.1. Infrared spectra, maxima of characteristic absorption bands of the most important atomic groups of the of Kara-Keche brown coal, humic acids and humine extracted from it
Comparative analysis of IR spectra of Kara-Keche brown coal, humic acids and humine showed that all samples contain condensed aromatic systems, aliphatic and alicyclic hydrocarbon groups, as well as oxygen-containing functional groups (carboxyl, carbonyl, hydroxyl).
All samples contain mineral components. The relative quantitative content of functional groups in the studied samples was estimated by the ratio of the peak areas of the absorption bands of oxygen-containing groups to the peak areas corresponding to aromatic polyconjugated systems (1600 cm–1) and aliphatic substituents at 2920 cm–1. The ratio of optical densities of absorption bands of alkyl substituents to aromatic fragments showed the predominance of aromatic fragments (A2920/A1600). Humic acids contain more alkyl substituents than humine. The relative amount of hydroxyl groups (A3400/A1600) in HA and HU is low compared to brown coal. Carboxyl groups (A1720/A1600) are much more prevalent in humic acids than in humine and coal.
From the data of Fig.1, it is also evident that in HA and HU, carboxyl groups are predominant over alkyl substituents, the ratio A1720/A2920 for both samples is greater than one. For coal the situation is opposite – the ratio A1720/A2920 is much less than unity. It can be stated that IR spectra of Kara-Keche coal, humic acids and humine extracted from it are similar. The main characteristic absorption maxima for humic substances are found in all samples. The differences are observed mainly in intensity – humic acids are dominated by oxygen-containing groups, while coal is dominated by alkyl substituents. More aromatic fragments are observed in the composition of humine. At the same time, differences in broadening and shifts of absorption bands of absorption spectra of Kara-Keche coal, humic acids and humic acid can be associated with both different chemical environment within the structural units of the studied objects and intermolecular interactions. Therefore, and taking into account the fact that the investigated carbon matrices are chemically heterogeneous supramolecular systems, comparative analysis of their IR spectra before and after adsorption will not provide objective information about the nature of the functional groups of coal, humine, and humic acids involved in the binding of urea, glycine, and D-glucose, respectively.
Scanning electron microscopy of Kara-Keche brown coal, humic acids and humine extracted from it. The analytical sample of Kara-Keche coal was examined on a TESCAN VEGA 3 LMH microscope with a secondary electron detector. The surface morphology of humine and humic acid samples was examined on a JEOL JSM 6510 scanning electron microscope using an SEI secondary electron detector and a BEC reflected electron detector.
Figure 2 shows photos of analytical samples of Kara-Keche brown coal, humic acids and humine extracted from it. The conglomerate of the raw coal sample has a lamellar structure, with a partially faceted structure observed for the constituent particles. Upon higher magnification of the sample surface, it can be seen that the coal has a poorly developed porous surface, due to which its negligible adsorption properties can be expected to manifest. Humic acid particles of quasi-crystalline form are grains of various sizes not exceeding 100 μm. The surface of humic acid particles is flat, not penetrated by pores and capillaries, without any kind of roughness.
Humine, in contrast to humic acids, are particles of irregular, splintered shape up to 100 μm. Particles with developed, porous surface are present. The maximum size of individual pores of humic acid reaches 200 nm.
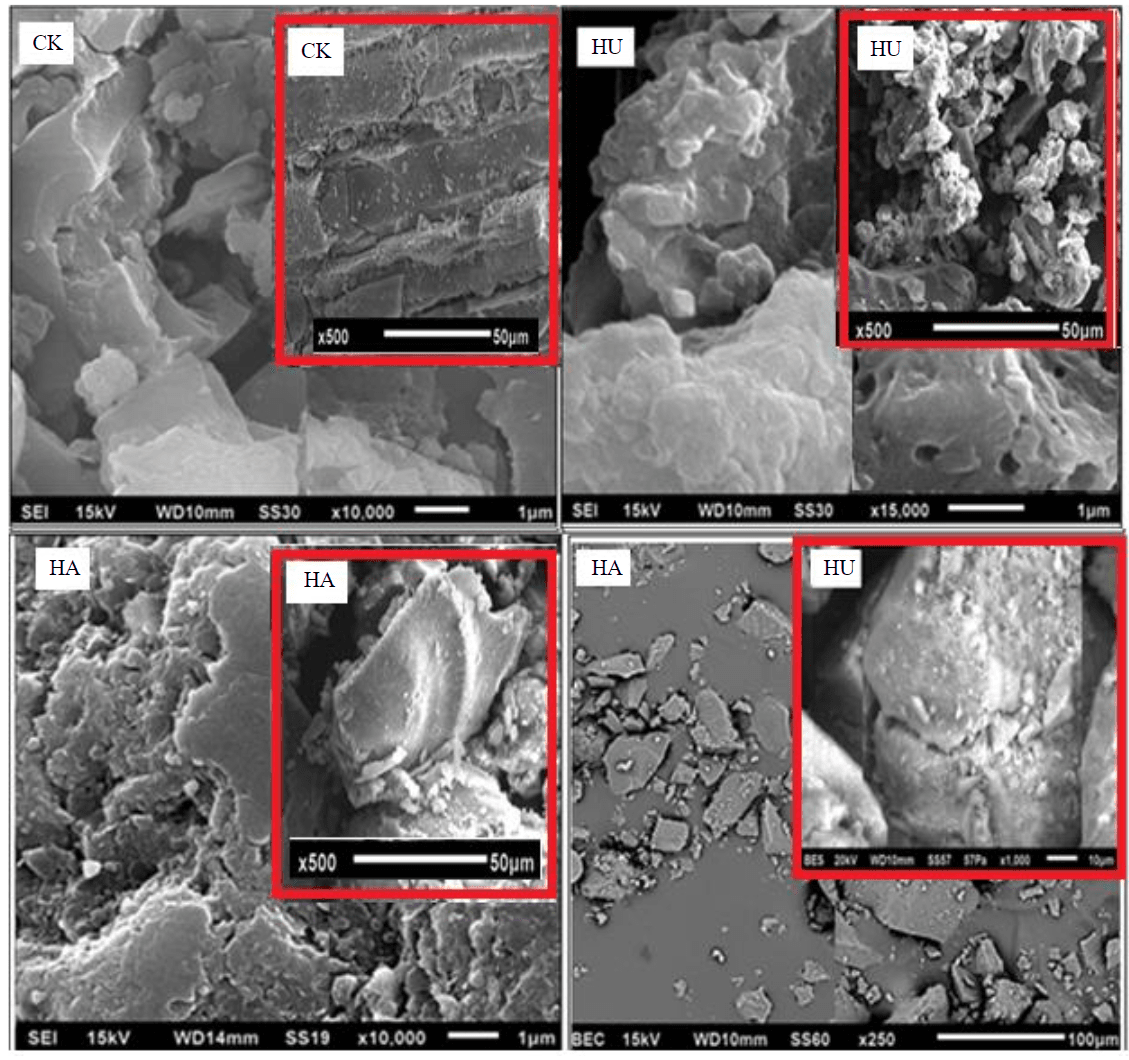
Fig.2. Surfaces of Kara-Keche brown coal, humic acids and humine extracted from it at different magnifications
Also shown in Fig.2 are photographs of humic acids and humin from Kara-Keche brown coal, taken using a reflected electron detector. The surface of humic acid and humine is homogeneous grey colour with rare small light inclusions representing more oxidized fragments in the structure of the studied substances or zones with increased content of ash elements.
As a result of the surface evaluation of the studied samples, considering only one physical aspect - the surface configuration, one can expect different adsorption mechanisms – from monomolecular in humic acid, to volumetric filling of micropores – in humine and coal.
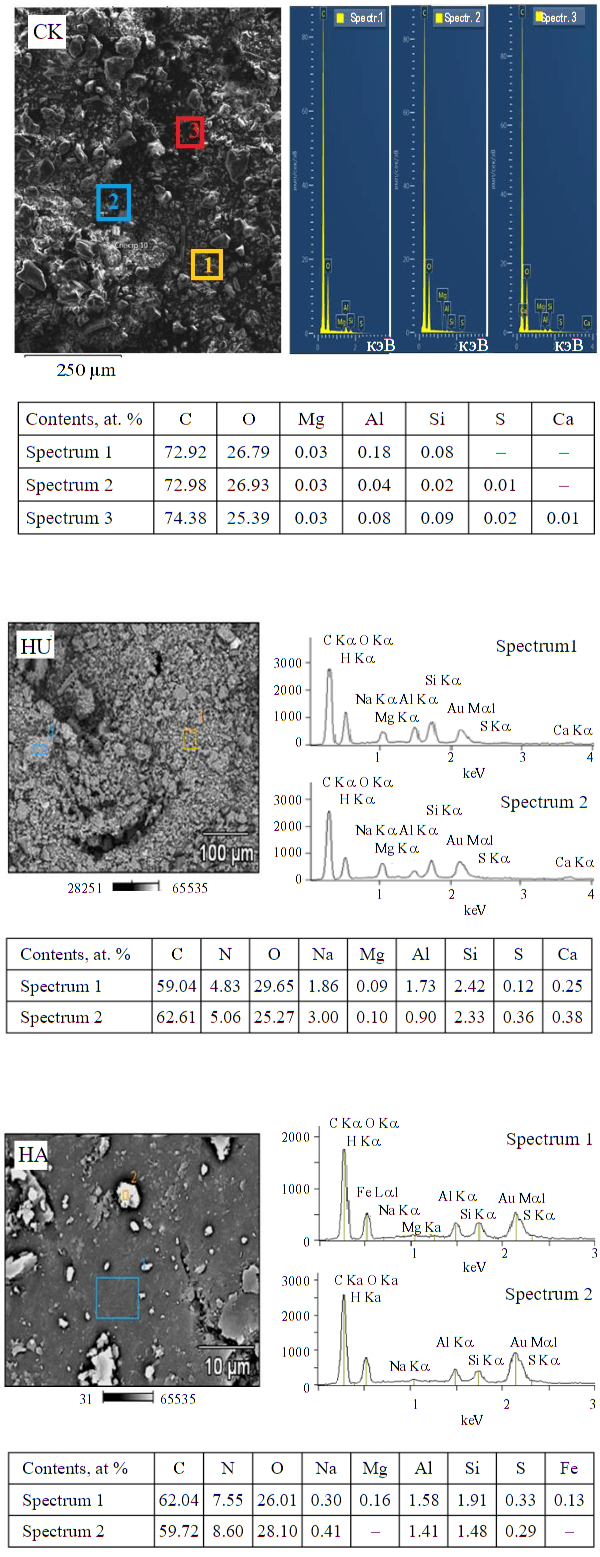
Fig.3. Energy dispersion spectra at given points on the surface of Kara-Keche brown coal, humic acids and humin extracted from it
Energy dispersive spectra of Kara-Keche coal, humic acids and humine extracted from it. The analysis of local elemental composition at given points of the studied samples of humic acids and humine was carried out on a JEOL JSM 6510 scanning electron microscope, a nitrogen-cooled energy dispersive spectrometer NSS7 was used as a detector. The local content of various elements in the Kara-Keche coal sample was determined on a TESCAN VEGA3 LMH microscope using an X-ACT EDS analyzer.
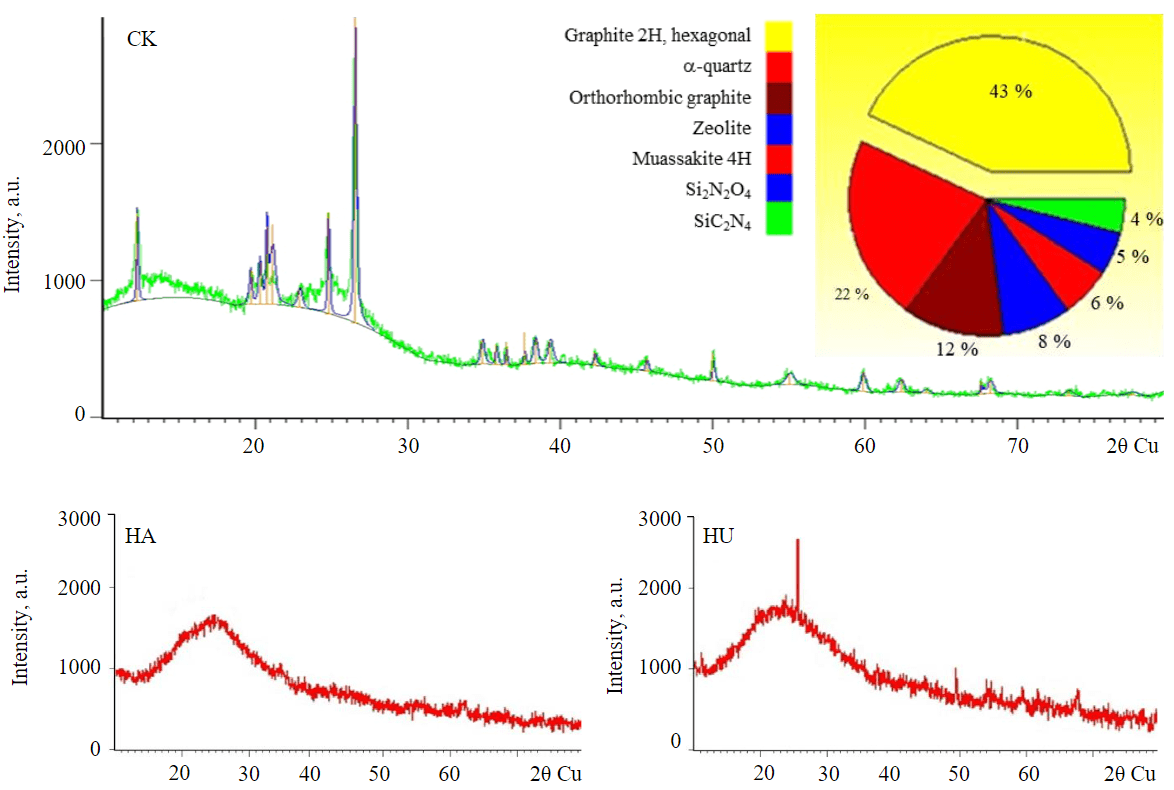
Fig.4. Diffraction spectra of Kara-Keche brown coal, humine and humic acids extracted from it
The study of the local elemental composition for all three samples was carried out in several morphologically different surface specimens. Data on the relative content of elements of the selected candidates, are shown in Fig.3. The quantitative content of elements at different points of the surface of the investigated samples of coal, humic acids and humine varies considerably (given in atomic per cent). The obtained result indicates that the studied samples of natural carbon matrices are chemically heterogeneous, dispersed, microheterogeneous supramolecular systems.
X-ray phase analysis of Kara-Keche coal powders, humine and humic acids extracted from it. An optimal set of methods for analyzing natural substances also includes X-ray phase analysis [39]. The powders of coal, humic acids and humine extracted from it were analysed on a Panalytical X'Pert Pro X-ray diffractometer (Philips) at the wavelength of copper Ka radiation λ = 0.154 nm.
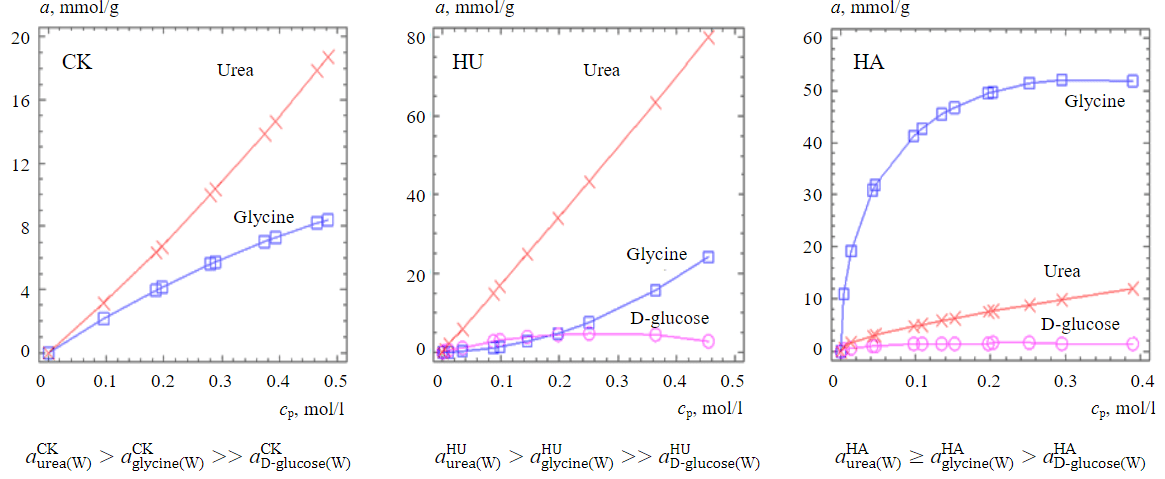
Fig.5. Isotherms of adsorption of urea, glycine, D-glucose from aqueous solutions on Kara-Keche brown coal, humic acids and humine extracted from it at 298 K
Figure 4 shows the diagram of mineral composition of Kara-Keche brown coal. The radiographs were recorded in GIXD geometry at 1° and 5° in the range of 20-80° (step 0.05°, time per step 2 s).
The analysis of the diffractograms and the search for candidate matches according to official PDF databases showed the presence of various modifications of graphite, silicon dioxide (quartz), aluminosilicates (zeolite), various carbides, carbide-nitrides and silicon nitride-oxides in the brown coal sample.
X-ray radiographs of humine and humic acids have the view of a broad line (halo) characteristic of amorphous materials (Fig.4). However, the presence of peaks in humine (residual coal) samples indicates the presence of structural formations similar to Kara-Keche brown coal.
Adsorption of low molecular weight organic compounds from aqueous solutions on Kara-Keche coal, humic acids and humine extracted from it. The adsorption studies used carbon sorbents whose particle sizes did not exceed 0.25 mm (60 mesh). Urea, glycine, D-glucose were chosen as adsorbates characterised by different type of variations from ideality in aqueous solutions. They are representatives of different classes of organic compounds that are of interest to specialists in biochemistry, biotechnology, ecology, and materials science. The bipolar molecules of urea and glycine are characterised by self-association in aqueous solutions, which determines their positive variations from ideality. Heteroassociation of the components of the aqueous solution of D-glucose underlying the mechanism of mutarotation of the monosaccharide suggests negative deviations from ideality in this binary system.
Conductivity and liquid chromatography methods were used to determine glycine concentrations in aqueous solutions. The polarimetric method was used to determine the concentration of D-glucose in aqueous solutions. The concentration of urea in aqueous solutions was determined chromatographically on a JASCO-HPLC high-efficiency liquid chromatograph.
Heterogeneous equilibrium in the system sorbent in solid phase – aqueous-organic solution was established during 24 h in dry-air thermostat at T = 298 K. The phases were stirred every 15 min for the first two hours. After 24 h the phases were separated on a syringe filter with a pore size of 1-2 µm. Adsorption values were calculated using the equation:
where c0 and cp – initial and equilibrium concentrations of adsorbate, M; V – volume of solution from which adsorption occurs, l; m – mass of adsorbent, g; 1000 – conversion factor of the obtained result in another unit of measurement – mmol/g.
The calculated adsorption values for each composition of the aqueous-organic solution were averages of three or four parallel tests with an accuracy of 10 % relative error.
The dependence of the above methods of determining the concentrations of urea, D-glucose, and glycine on the acidity of the medium allowed us to conclude that there were no noticeable changes in the acid-base manifestation in the studied systems after adsorption.
Visualization of experimental data of isothermal adsorption is presented in Fig.5. The series on adsorption of urea, glycine, D-glucose from aqueous solutions on Kara-Keche brown coal, humic acids and humin extracted from it, respectively, are shown. Differences are observed for adsorption of glycine and urea on humic acids compared to brown coal and humine. In this case, the adsorption of D-glucose from aqueous solution on coal was found to be negligible. It can be assumed that for bipolar molecules of urea and glycine both physical adsorption and chemosorption on brown coal, humic acids and humine extracted from it are realised.
For a neutral monosaccharide molecule, only the formation of weak hydrogen bonds can be expected with the adsorption centres of all investigated carbon sorbents.
The reversal of the series for adsorption of urea and glycine on humic acids is due to the binding of glycine to the surface carboxyl groups of the carbon matrix of the sorbent matrix. The greater polarity of urea compared to glycine obviously affects its preferential adsorption in the pores, roughness of brown coal and humine.
In order to evaluate possible adsorption mechanisms in the studied systems, the data of Fig.5 are considered in the framework of ideas about monomolecular adsorption and volumetric filling of sorbent micropores using the Langmuir and Dubinin – Radushkevich equations. The results of calculations are given in Table 2.
Linearisation of experimental data with high correlation coefficients on adsorption of investigated organic compounds from aqueous solutions on lignite, humine extracted from it is provided in coordinates of the Dubinin – Radushkevich equation, and for humic acids – in coordinates of the Langmuir equation.
Table 2
Adsorption of urea, glycine, D-glucose from aqueous solutions on Kara-Keche brown coal, humic acids and humine extracted from it at 298 K in coordinates of the Langmuir and Dubinin – Radushkevich equations
|
Sorbent |
Sorbat |
The equations of linear regression in coordinates of Dubinin – Radushkevich and Langmuir equations |
Correlation coefficient kcorr |
Adsorption а∞, mmol/g |
|
CK |
Urea |
lna = 7.47 + 1.21ln(cр/20) |
0.99 |
1752.7 |
|
Glycine |
lna = 3.20 + 071ln(cр/3.4) |
0.78 |
24.6 |
|
|
HU |
Urea |
lna = 9.17 + 1.17ln(cр/20) |
1.00 |
9604.6 |
|
Glycine |
lna = 8.91 + 2.80ln(cр/3.4) |
0.94 |
7405.7 |
|
|
D-glucose |
lna = 1.85 + 0.08ln(cр/4.7) |
0.98 |
6.4 |
|
|
HA |
Urea |
cр/a = 0.009 + 0.105cр |
1.00 |
9.5 |
|
Glycine |
cр/a = 0.0003 + 0.018cр |
1.00 |
55.5 |
|
|
D-glucose |
cр/a = 0.011 + 0.610cр |
1.00 |
1.6 |
The obtained result suggests adsorption of low-molecular organic compounds from aqueous solutions on humic and coal mainly in irregularities and pores of carbon matrices of the studied sorbents. On humic acids, on the contrary, the binding of the considered sorbates is provided mainly on their surface reaction centres. It is obvious that adsorption of urea, glycine, D-glucose from aqueous solutions on brown coal, humic acids and humine extracted from it is caused by a number of forces, including physical and chemical interactions of sorbate molecules with functional groups of carbon matrices of the studied sorbents.

Fig.6. Specific surface area of Kara-Keche brown coal, humic acids and humine extracted from it, determined by glycine, urea and D-glucose
The obtained values of the limiting adsorptions of urea, glycine, D-glucose from aqueous solutions on Kara-Keche brown coal, humic acids and humine extracted from it were used to estimate the specific surface area of these carbon sorbents. For this, using the 3D demo version of Chemical Office, the area occupied by sorbate molecules on the solid surface was calculated (Fig.6). From the histogram of Fig.6, it can be seen that humene has the highest specific surface area compared to humic acids and coal. Obviously, the result obtained is due to the developed, porous structure of the humin established by morphological studies.
Conclusion
The studied natural carbon matrices – Kara-Keche brown coal, humic acids and humine extracted from it – are amorphous, chemically heterogeneous supra-molecular systems with structural formations.
On humic and Kara-Keche brown coal, volumetric filling of sorbent micropores with urea, glycine, D-glucose from aqueous solutions is carried out. Surface adsorption of investigated low molecular weight organic compounds from aqueous solutions takes place on humic acids.
In the range of considered natural carbon matrices, humine has the highest limiting adsorption capacity with respect to urea, glycine and D-glucose from their aqueous solutions. Due to the developed pore structure, stability in acids and alkalis, humine of Kara-Keche brown coal can be recommended as an effective sorbent for treatment of industrial wastewater from low molecular weight organic compounds.
References
- Zherebtsov S.I., Malyshenko N.V., Votolin K.S. et al. Structural-Group Composition and Biological Activity of Humic Acids Obtained from Brown Coals of Russia and Mongolia. Solid Fuel Chemistry. 2019. Vol. 53. N 3, p. 145-151. DOI: 10.3103/S0361521919030121
- Votolin K.S., Zherebtsov S.I., Shpakodraev K.M. Physicochemical Studies of the Humic, Hymatomelanic and Fulvic Acids of Brown Coals. Chemistry for Sustainable Development. 2023. Vol. 31. N 5, p. 480-489. DOI: 10.15372/CSD2023493
- Pankratov D.A., Anuchina M.M., Konstantinov A.I., Perminova I.V. Analyzing the Dynamics of Interaction between Humic Coal Substances and Metallic Iron. Russian Journal of Physical Chemistry A. 2019. Vol. 93. N 7, p. 1235-1244. DOI: 10.1134/S0036024419070203
- Zenkov I.V. Foreign economic cooperation of countries with coal power generation on the electrical energy market in Eastern Europe. Ugol. 2020. N 11 (1136), p. 71-73 (in Russian). DOI: 10.18796/0041-5790-2020-11-71-73
- Olk D.C., Bloom P.R., Perdue E.M. et al. Environmental and Agricultural Relevance of Humic Fractions Extracted by Alkali from Soils and Natural Waters. Journal of Environmental Quality. 2019. Vol. 48. Iss. 2, p. 217-232. DOI: 10.2134/jeq2019.02.0041
- Zavarzina A.G., Danchenko N.N., Demin V.V. et al. Humic Substances: Hypotheses and Reality (a Review). Eurasian Soil Science. 2021. Vol. 54. N 12, p. 1826-1854. DOI: 10.1134/S1064229321120164
- Zhong-Ting Hu, Weizhong Huo, Yue Chen et al. Humic Substances Derived From Biomass Waste During Aerobic Composting and Hydrothermal Treatment: A Review. Frontiers in Bioengineering and Biotechnology. 2022. Vol. 10. N 878686. DOI: 10.3389/fbioe.2022.878686
- Likhacheva N.A., Mitrofanova V.V. Sorption of heavy metal ions by humic substances. Bashkir Chemistry Journal. 2022. Vol. 29. N 4, p. 41-48 (in Russian). DOI: 10.17122/bcj-2022-4-41-48
- Chukaeva M.A., Puhalsky J.V., Loskutov S.I. et al. Assessment of changes in the heavy-metal phytoextraction by Tagetes erecta from contaminated soils of Norilsk using humic additives. Arctic: Ecology and Economy. 2024. Vol. 14. N 1, p. 90-102 (in Russian). DOI: 10.25283/2223-4594-2024-1-90-102
- Jianguo Cheng, Shanfei Zhang, Chen Fang et al. Removal of Heavy Metal Ions from Aqueous Solution Using Biotransformed Lignite. Molecules. 2023. Vol. 28. Iss. 13. N 5031. DOI: 10.3390/molecules28135031
- Liwen Zheng, Yongchao Gao, Jianhua Du et al. Single and Binary Adsorption Behaviour and Mechanisms of Cd2+, Cu2+ and Ni2+ onto Modified Biochar in Aqueous Solutions. Processes. 2021. Vol. 9. Iss. 10. N 1829. DOI: 10.3390/pr9101829
- Karabaev S.O., Subankulova D.A., Gainullina I.P., Djunushalieva A.K. Binding processes of copper, nickel, cadmium ions on humin and humic acid of KaraKeche coal. Bulletin of the KNU named after Zhusupa Balasagyna. 2018. N 4 (96), p. 125-130 (in Russian).
- Dzhunushalieva A.K. Selective solvation and adsorption of heavy metal ions from aqueous solutions on humic acid, humine of brown coal: Avtoref. dis … kand. khim. nauk. Bishkek: Kyrgyzskii natsionalnyi universitet imeni Zhusupa Balasagyna, 2022, p. 23 (in Russian).
- Lisichkin G.V., Kulakova I.I. Elimination of Emergency Oil Spills: State of the Art and Problems. Russian Journal of Applied Chemistry. 2022. Vol. 95. N 9, p. 1263-1289. DOI: 10.1134/s1070427222090014
- Hongxiang Xu, Shan Li, Jingzheng Wang et al. Removal of Pyridine from Aqueous Solutions Using Lignite, Coking Coal, and Anthracite: Adsorption Kinetics. Processes. 2023. Vol. 11. Iss. 11. N 3118. DOI: 10.3390/pr11113118
- Chukaeva M.A., Zaytseva T.A., Matveeva V.A., Sverchkov I.P. Purification of Oil-Contaminated Wastewater with a Modified Natural Adsorbent. Ecological Engineering & Environmental Technology. 2021. Vol. 22. № 2. P. 46-51. DOI: 10.12912/27197050/133331
- Cheremisina O., Litvinova T., Sergeev V. et al. Application of the Organic Waste-Based Sorbent for the Purification of Aqueous Solutions. Water. 2021. Vol. 13. Iss. 21. N 3101. DOI: 10.3390/w13213101
- Cheremisina O.V., Ponomareva M.A., Molotilova A.Yu. et al. Sorption purification of acid storage facility water from iron and titanium on organic polymeric materials. Journal of Mining Institute. 2023. Vol. 264, p. 971-980. DOI: 10.31897/PMI.2023.28
- Grigorieva Е. About humic preparations. International Agricultural Journal. 2020. Vol. 63. N 5, p. 40-54 (in Russian). DOI: 10.24411/2588-0209-2020-10210
- Fisha P.C., Budina E.V., Zherebtsov S.I. et al. Comparative assessment of the use of humic substances derived from brown coals for technogenic landscapes reclamation. Journal of Soils and Environment. 2021. Vol. 4. N 1, p. 10 (in Russian). DOI: 10.31251/pos.v4i1.135
- Koshelev A.V., Golovkov V.F., Derevyagina I.D. et al. Research of humic preparations obtained from lignite. Chemistry and Technology of Organic Substances. 2019. N 3 (11), p. 28-40 (in Russian). DOI: 10.54468/25876724_2019_3_28
- Lesnikova E.B., Artemova N.I. Technology for the Production and Use of Humic Preparations for Ecological Purposes. Solid Fuel Chemistry. 2019. Vol. 53. N 6, p. 332-338. DOI: 10.3103/S0361521919060065
- Alghamdi A.A., Al-Odayni A.-B., Saeed W.S. et al. Efficient Adsorption of Lead (II) from Aqueous Phase Solutions Using Polypyrrole-Based Activated Carbon. Materials. 2019. Vol. 12. Iss. 12. N 2020. DOI: 10.3390/ma12122020
- Skugoreva S.G., Kantor G.Ya., Zhukova A.V. The use of mathematical models to assess the sorption abilities of higher mushrooms and activated carbon in relation to copper (II) ions. Theoretical and Applied Ecology. 2020. N 2, p. 44-50 (in Russian). DOI: 10.25750/1995-4301-2020-2-044-050
- Jianlong Wang, Xuan Guo. Adsorption kinetic models: Physical meanings, applications, and solving methods. Journal of Hazardous Materials. 2020. Vol. 390. N 122156. DOI: 10.1016/j.jhazmat.2020.122156
- Wells M.J.M., Stretz H.A. Supramolecular architectures of natural organic matter. Science of the Total Environment. 2019. Vol. 671, p. 1125-1133. DOI: 10.1016/j.scitotenv.2019.03.406
- Klučáková M., Věžníková K. Micro-organization of humic acids in aqueous solutions. Journal of Molecular Structure. 2017. Vol. 1144, p. 33-40. DOI: 10.1016/j.molstruc.2017.05.012
- Tsareva A.A., Litvinova T.E., Gapanyuk D.I. et al. Kinetic Calculation of Sorption of Ethyl Alcohol on Carbon Materials. Russian Journal of Physical Chemistry A. 2024, p. 10. DOI: 10.1134/S0036024424030312
- Zhakina A.H., Vassilets Ye.P., Arnt O.V. et al. Template amino-humic sorbent based on coal mining waste. Universum: khimiya i biologiya. 2021. N 11 (89). Part 2, p. 41-45 (in Russian). DOI: 10.32743/UniChem.2021.89.11.12512
- Zykov I.Yu., Ivanov N.N., Fedorova N.I., Ismagilov Z.R. Study of formaldehyde adsorption by composite sorbents based on lignite and manganese oxide. Bulletin of the Kuzbass State Technical University. 2021. N 5 (147), p. 57-63 (in Russian). DOI: 10.26730/1999-4125-2021-5-57-63
- Vrantsi E., Lakka A., Bozinou E. et al. Humic and Fulvic Acids as Specific Sorbents of Herbicides in Water. Clean – Soil, Air, Water. 2021. Vol. 49. Iss. 11. N 2000467. DOI: 10.1002/clen.202000467
- Dzheldybaeva I.M., Kairbekov Zh.K., Maloletnev A.S. et al. Physicochemical and Antioxidant Properties of Humic Substances from Coals of the Oy-Karagay and Kiyakty Deposits in the Republic of Kazakhstan. Solid Fuel Chemistry. 2022. Vol. 56. N 6, p. 471-477. DOI: 10.3103/s0361521921060033
- Sverchkov I.P., Matveeva V.A., Chukaeva M.A. Determination of carbon, oxygen, hydrogen and nitrogen content in coals using WDXRF scattering spectra. Spectrochimica Acta Part B: Atomic Spectroscopy. 2023. Vol. 207. N 106738. DOI: 10.1016/j.sab.2023.106738
- Karayiğit A.İ., Oskay R.G., Bircan C. Mineralogical, Petrographical and Geochemical Properties of the Late Oligocene Coal Seam (Seam-VI): Insights into Elemental Enrichments and Palaeodepositional Environment (İbrice field, Thrace Basin). Yerbilimleri. 2024. Vol. 45. Iss. 1, p. 1-51. DOI: 10.17824/yerbilimleri.1393877
- Kopp D.D., Portnova A.V., Farberova E.A. Development of brown coal conversion methods. Bulletin of the Perm National Research Polytechnic University. Chemical Technology and Biotechnology. 2019. N 4, p. 133-146 (in Russian). DOI: 10.15593/2224-9400/2019.4.12
- Avgushevich I.V., Sidoruk E.I., Bronovets T.M. Standard test methods for coals. Classifications of coals. Moscow: Reklama Master, 2019, p. 576 (in Russian).
- Wei Wang, Long Liang, Yaoli Peng, Maria Holuszko. Surface Chemical Heterogeneity of Low Rank Coal Characterized by Micro-FTIR and Its Correlation with Hydrophobicity. Minerals. 2021. Vol. 11. Iss. 3. N 239. DOI: 10.3390/min11030239
- Jingyu Jiang, Weihua Yang, Yuanping Cheng et al. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification. Fuel. 2019. Vol. 239, p. 559-572. DOI: 10.1016/j.fuel.2018.11.057
- Sverchkov I.P., Gembitskaya I.M., Povarov V.G., Chukaeva M.A. Method of reference samples preparation for X-ray fluorescence analysis. Talanta. 2023. Vol. 252. N 123820. DOI: 10.1016/j.talanta.2022.123820
