Promising reagents for the extraction of strategic metals from difficult-to-enrich mineral raw materials
- 1 — Ph.D., Dr.Sci. Deputy Director Institute of Comprehensive Exploitation of Mineral Resources of the RAS ▪ Orcid
- 2 — Researcher Institute of Comprehensive Exploitation of Mineral Resources of the RAS ▪ Orcid
- 3 — Researcher Institute of Comprehensive Exploitation of Mineral Resources of the RAS ▪ Orcid
Abstract
The need of the mining and processing industry for new types of directional reagents is due to the deterioration of the material composition of the processed ores. Low Au content (less than 0.5-1.0 g/t), finely dispersed Au inclusions (0.1-10.0 microns) in the ore, similar properties of the separated minerals have an extremely negative effect on flotation performance when using traditional reagents, which leads to significant losses of valuable metal with enrichment tailings. Expanding the range of domestic flotation reagents based on the latest achievements of fundamental research and their targeted application at mining and processing companies will compensate for the negative impact of the mineral composition of raw materials and ensure maximum extraction of strategic metals from difficult-to-enrich ores. The use of modern research methods (scanning electron and laser microscopy, UV spectrophotometry, XRF and chemical analysis) made it possible to visualize the adsorption layer of new reagents-collectors of a number of dithiocarbamates with different structures of a hydrocarbon radical and an organic modifier on the surface of gold-containing sulfides. The amount of adsorbed reagents on the surface of minerals has been experimentally determined. The specific features of the fixation of reagents on minerals of various compositions led to optimal correlations of their consumption in the flotation process. Scientifically based reagent regimes ensured an increase in the gold content in the concentrate and a decrease in the loss of gold with tailings by 5-6 % during flotation enrichment of the refractory ore of the Malinovskoe deposit.
Funding
The research was carried out at the expense of a grant from the Russian Science Foundation N 22-17-00149, https://rscf.ru/en/project/22-17-00149/.
Introduction
Difficult-to-enrich mineral raw materials, including low-grade ores and man-made resources, are an important source of strategic metals. There is an imbalance in the mining and processing enterprises of the country caused by a discrepancy between the available raw material base and the level of mineral processing technology development, which leads to significant losses of useful components, reaching 30 % for traditional ores and significantly more for hard-to-recover and man-made resources [1].
In conditions of a constant decrease in the content of valuable metals in the feedstock and the subtle mutual undergrowth of ore minerals and host rocks, new technologies are being developed for more efficient extraction of strategic metals. These technologies are based on combined schemes, where flotation plays an important role [2-5].
Existing flotation reagents have their limitations and do not always provide high enrichment rates. Therefore, research is underway to develop new flotation chelating reagents that can be more effective and cost-effective. Such studies include not only the synthesis of new compounds, but also the study of their properties, the determination of optimal application conditions, as well as an assessment of their impact on the technological parameters of the flotation process.
The use of classical reagent modes often leads to a decrease in the quality of concentrates and an increase in the cost of final products. To reduce economic costs, it is necessary to use new environmentally friendly domestic reagents that can significantly increase the extraction of valuable metals from difficult-to-enrich mineral raw materials [6-8].
A wide range of organic and inorganic reagents is used for flotation [9-12]. Some reagents have passed the test of time [13, 14], while new ones reflect the changing needs of the industry [15-17]. New and improved reagents meet both technological and environmental requirements [18-20].
The Russian market of flotation reagents is characterized by the presence of large domestic manufacturers such as OOO Kvadrat Plus, AO Volzhsky Orgsintez, OOO MBI-Sintez, OOO Flotent Chemicals Rus, OOO NPP QVALITET, AO UK BHH Orgkhim. The product range includes dithiophosphates [11], xanthogenates, dithiocarbamates, thionocarbamates [21] and other reagents. Despite the large assortment, xanthogenates traditionally occupy a leading position in processing plants.
In modern conditions of intensification of flotation enrichment and processing of complex multicomponent ores, there is an urgent need to develop and introduce new collecting reagents with increased efficiency and selectivity.
Research in this area is conducted by the Empress Catherine II Saint Petersburg Mining University [8, 9], the National Research Technological University “MISIS” [22-24], the Institute of Mining SB RAS [25, 26], the Mining Institute of the KSC RAS [27], Irkutsk National Research Technical University [28], the All-Russian Scientific-Research Institute of Mineral Raw Materials named after N.M.Fedorovsky [29, 30]. The compositions of selective collectors based on thionocarbamates contributed to an increase in the extraction of gold from pyrite copper-zinc ores [22, 23]. Acetylene alcohols and reagents based on them have been proposed for the recovery of gold from porphyry copper ores [24].
The kinetics of the interaction of collectors with minerals has been studied and the role of the physical form of sorption of the reagent in the elementary act of flotation has been shown [25, 26]. Research is being conducted on choosing the selective collectors for the extraction of minerals of non-ferrous and rare metals from complex ores (RF Patent N 2381073). Foreign researchers propose the use of humate and hypochlorite for the depression of arsenopyrite and the separation of chalcopyrite from galenite [31, 32], butylaminocellulose in the flotation of sulfide ores [33], various combinations of sulfhydryl collectors [34-36] and organic modifiers [37] for the flotation of polymetallic ores.
Under the leadership of Academician V.A.Chanturia, the ICEMR RAS conducted systematic research on the development and testing of new effective targeted reagents for the extraction of gold, platinum, copper and other valuable metals from difficult-to-enrich mineral raw materials [15, 38, 39].
Water solutions of the salt of dithiocarbamic acid – morpholindithiocarbamate sodium (morpholineDTC) and cyanethyldiethyldithiocarbamate (CEDEDTC) were tested both when individually fed into flotation and in a composition with potassium butyl xanthogenate (PBX). Hogweed extract (HE) has been studied as a surface modifier of gold-containing sulfides. The listed reagents have the ability to complex, due to which they have a selective effect on the floatability of gold-containing sulfides [40, 41].
Hogweed extract contains tannin, essential oils, coumarins in addition to carbohydrates, sugar, proteins, carotene, and amino acids [34]. Of greatest interest are the coumarins included in the hogweed, which are cyclic compounds with chemically active groups capable of forming complex compounds with metals.
The purpose of this work was to obtain new scientific knowledge about the mechanism of action of chelating reagents-collectors – derivatives of dithiocarbamates and an organic modifier on gold-containing sulfide minerals and to substantiate the prospects of their use individually and in combination with butyl xanthogenate to increase the extraction of strategic metals from difficult-to-enrich mineral raw materials.
Methods
Scanning of the surface of gold-bearing sulfide minerals and ore samples of the Malinovskoe deposit was performed by laser (KEYENCE VK-9700) and electron (LEO 1420VP) microscopy. The complex of these methods made it possible to identify the most characteristic areas and evaluate the difference in images before and after interaction with reagent solutions. The shape of the adsorbed organic phase of the reagent on the surface of minerals is visualized on the images of electron and laser microscopes and its dimensions are determined.
During the study, the linear dimensions and areas of reagent growths on the surface of minerals were measured. The images of the sulfide anslides surface obtained on a laser microscope after treatment with a reagent were analyzed according to the method [42]. The technique is based on measuring the area of the sites occupied by the reagent and calculating the degree of coating of the mineral with the reagent.
The ore of the Malinovskoe deposit belongs to the gold sulfide-quartz type, in which Au (1.4 g/t), Ag (50.3 g/t) and Cu (1.3 %) represent the main industrial value. Sulfide minerals are represented by FeAsS, FeS2, CuFeS2 and FenSn+1. Less common are MoS2, CaWO4, ZnS, PbS, FeO·Fe2O3, native Au. The average amount of sulfides is 10-15 %. The content in the ore is As – 1, Fe – 8.6, Pb – 0.05, W – 0.08 %. Gold is associated with chalcopyrite (CuFeS2) and arsenopyrite (FeAsS) [43].
According to the chemical analysis of the ME-ICP06 ore sample, the maximum content of oxide minerals is represented by quartz (more than 60 % SiO2), about 14 % Al2O3, about 20 % total iron, potassium and magnesium oxides. According to the results of X-ray phase analysis, the main rock-forming minerals in the sample are quartz – 67.2 %, chlorite – 11.5 %, muscovite – 7.5 %.
The analytical complex Mineral C7 (Olympus BX51 optical microscope) was used for minera-logical description. Mineral C7 allows to automatically determine the mineral composition of the sample, the grain size of the mineral and the mass fraction of minerals in the aggregates. Anschlift studies and microphotography were carried out using a software and hardware complex of a laser microscope.
The characteristic maxima of light absorption and the concentration of reagents in solution were determined by UV spectroscopy. The amount of the adsorbed reagent on the mineral was estimated after mixing its solution with a crushed fraction of the mineral with a grain size of –0.1+0.063 mm according to the formula
where Vl.p. – the volume of the liquid phase, ml; Cin – the initial reagent concentration, mg/l; Cres – the residual reagent concentration, mg/l; P – the weight of the mineral sample, g.
Flotation conditions of the Malinovskoe ore deposit: an ore sample, crushed to a size of 90 % – 0.071 mm, was placed in the chamber of a laboratory flotation machine FML 1 (237 FL). Flotation took place for 5 min with variable reagent consumption.
The gold content in the samples after flotation was assessed by assay analysis followed by determination of Au by atomic absorption spectrometry in the accredited laboratory of LLC Stewart Geochemical and Assay.
Discussion of results
The UV spectroscopy method has shown the possibility of the formation of chelate complexes of new reagents with gold. Ultraviolet (UV) spectroscopy is widely used to study chemical reactions, including the interaction of various reagents with gold. In this case, the absorption spectra of the studied solutions in the UV range are analyzed (Table 1).
Table 1 shows the wavelengths (λmax), at which the maxima of light absorption of the studied chemical compounds and the values of optical densities (D), measured on a spectrophotometer are determined. When mixing a water solution of morpholindithiocarbamate and a gold-containing solution, significant changes in the electronic spectrum are observed. The morpholineDTC UV spectrum has characteristic light absorption peaks at 261.2 and 285.2 nm. After its interaction with hydrogen tetrachloroaurate(III), new maxima λmax – 275.6 and 313.5 nm, which are absent in the electronic spectra of the initial solutions, are determined.
Table 1
The results of the analysis of UV spectra of solutions of the studied chemical compounds
|
Chemical compound |
λmax, nm |
D |
|
Hydrogen tetrachloroaurate(III) |
223.1 302.4 |
0.56 0.73 |
|
MorpholineDTC |
261.2 285.2 |
0.39 0.41 |
|
MorpholineDTC Complex with Au |
275.6 313.5 |
0.62 0.55 |
|
CEDEDTC |
223.0 251.7 274.3 |
0.38 0.45 0.52 |
|
CEDEDTC Complex with Au |
254.3 318.1 |
0.47 0.16 |
In the electronic spectrum of the studied mixture, there is a change in the values of optical densities, which differ from the additive sum D of the initial substances, which means that an interaction occurs between the components, leading to the formation of a morpholineDTC complex with Au.
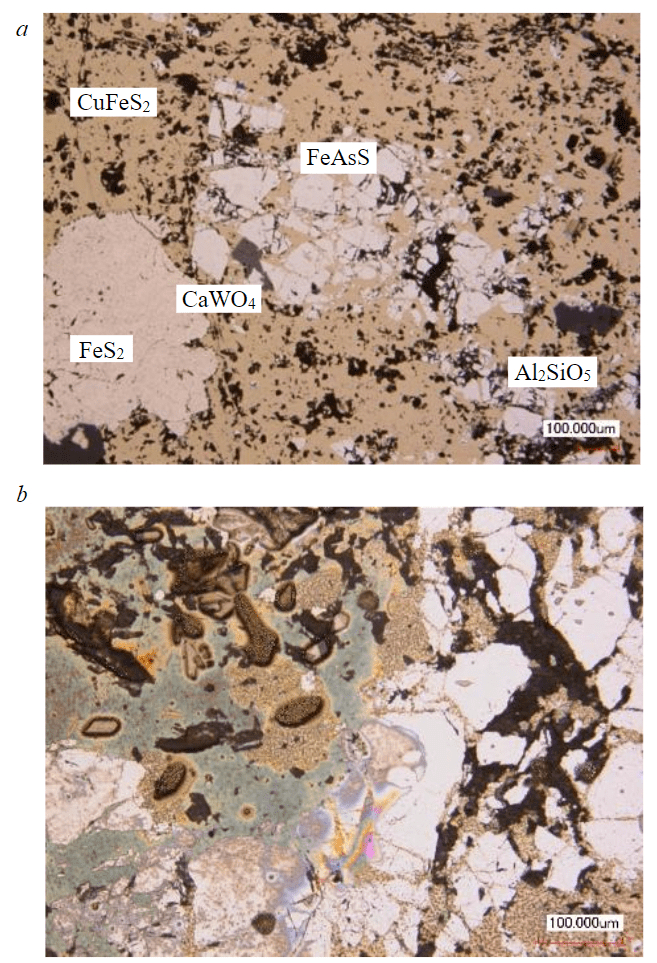
Fig.1. Native ore sample (а) and after treatment with morpholineDTC and HE (b)
Similar changes are observed in the interaction of CEDEDTC with a gold-containing solution. The initial cyanethidiethyldithiocarbamate solution is characterized by peaks of λmax = = 251.7 and 274.3 nm. After the addition of hydrogen tetrachloroaurate(III), new peaks appear at wavelengths of 254.3 and 318.1 nm, at the same time, the maximum light absorption disappears at λmax = 274.3 nm. These data confirm the formation of the CEDEDTC complex with Au.
The adsorption of morpholineDTC on the surface of minerals with artificially deposited Au was studied on fractions of sulfides crushed to a size of –0.1+0.063 mm. The evaluation criterion was a change in the concentration of the reagent, which was calculated using UV spectra.
It was experimentally established that after 5 min of conditioning of a morpholinedithiocarbamate solution (C = 20 mg/l) with gold-containing arsenopyrite and chalcopyrite, its concentration in the suspension drops to 2.5 mg/l, which confirms the fact of adsorption of morpholineDTC on the surface of sulfides. Calculations have shown that the adsorption of morpholindithiocarbamate is 0.7 mg/g, i.e. 87.5 % of morpholineDTC is fixed on minerals.
UV spectroscopy data are necessary to understand the mechanisms of interaction of gold with the reagents morpholineDTC and CEDEDTC, as well as adsorption processes on gold-bearing minerals, which is important for the development of methods for extracting gold from ores.
Laser microscopy was used to obtain images (Fig.1) of the surface of the initial ore anshliff of the Malinovskoe deposit and after its conditioning with solutions of morpholindithiocarbamate and hogweed extract. There is a different nature of the adsorption of reagents on the minerals of the ore sample: an intermittent light brown film of the morpholineDTC reagent was distributed over the entire surface of CuFeS2, and brown growths appeared on FeS2, morpholineDTC has not been fixed on other ore minerals. A pale blue film of HE plant extract was fixed “on top” of morpholineDTC on chalcopyrite and pyrite. The energy dispersion spectrum of the HE film on minerals is identical to the spectrum of the initial extract. Earlier, the individuality of the formation of the adsorption layer of chelating reagents was also noted on monomineral anschlifts. The newly formed phases persist after rinsing the anschlift with water, which confirms the stable fixation of the reagent on the surface.
The laser microscope images of the surface of the FeAsS, CuFeS2 and CuFeS2 anschlifts with Au deposited after treatment with the CEDEDTC reagent were analyzed according to the method [42]. The results of image analysis showed that the value of the degree of surface coating of minerals with cyanethyldiethyldithiocarbamate is 8 % for FeAsS, 12 % for CuFeS2 and 20.8 % for CuFeS2 with Au deposited.
In experiments conducted under the same conditions, it was found that the degree of coating varies and can be used as a basis for selective separation during flotation. It is worth noting that chalcopyrite with Au has the highest surface coverage area among the studied minerals, i.e. it has a greater ability to adsorb the reagent, which may be useful when extracting it from ore.
After contact of chalcopyrite and arsenopyrite with CEDEDTC solution, teardrop-shaped growths appeared on their surface (Fig.2, a). During the subsequent processing of minerals with hogweed extract, the surface is covered with an iridescent film (Fig.2, b).
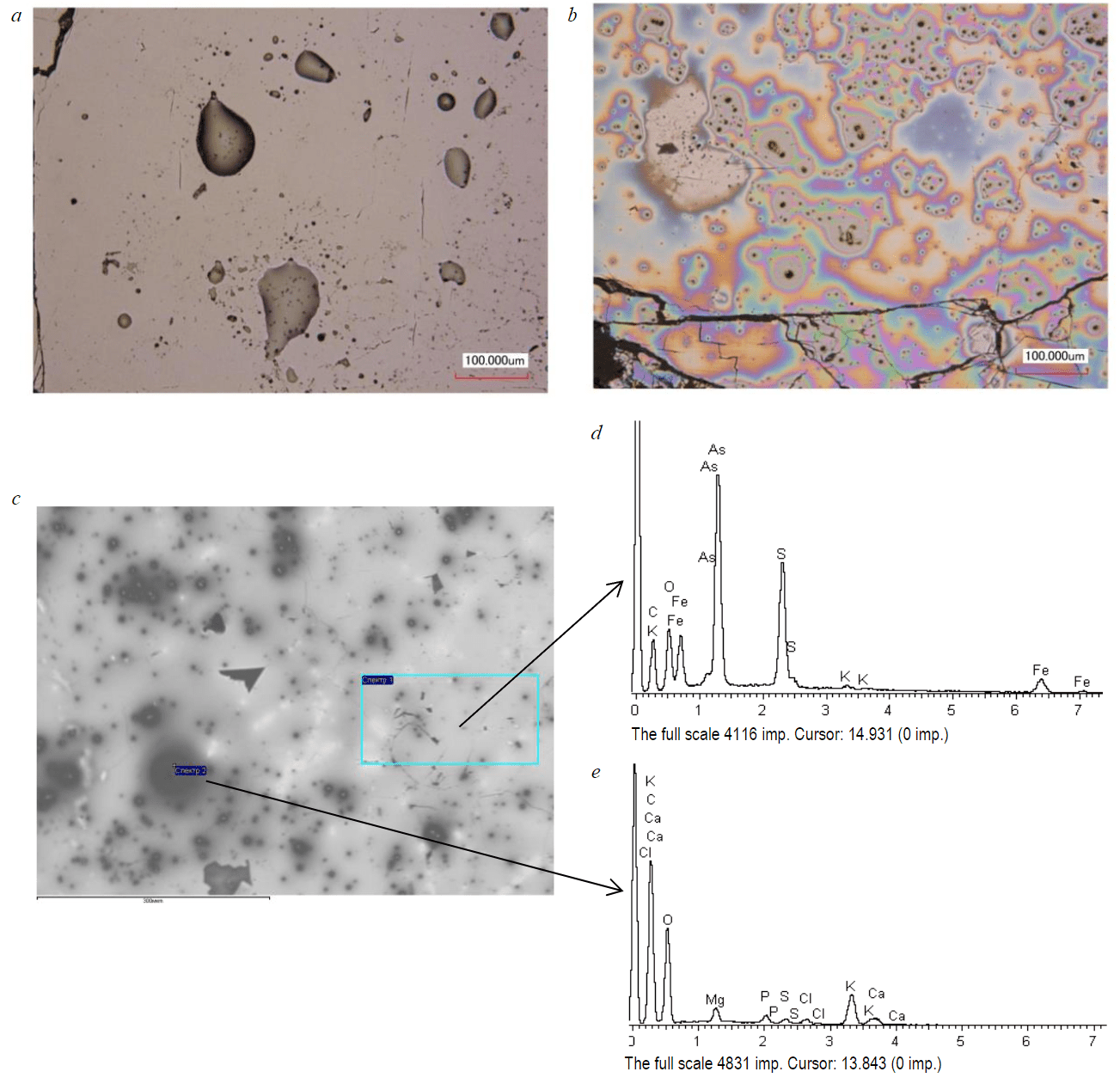
Fig.2. The surface of arsenopyrite after interaction: a – with CEDEDTC; b, c – with CEDEDTC and HE; d, e – energy dispersion spectra
Micrographs of the LEO 1420VP electron microscope on the FeAsS surface show black islands (Fig.2, c), the energy dispersion spectrum of which (Fig.2, d, e) coincides with the HE spectrum. In addition to the peaks of carbon C and oxygen O, intense peaks of K and Ca, as well as weak peaks of Mg, P, S and Cl, were found in the energy dispersion spectrum of HE.
A comprehensive study of the nature of the inclusions of ore minerals and low-dimensional mineral aggregates in the studied mineral samples allowed to form an idea of the types of mineral associations and morphological features of the grains. At the same time, the determining effect of the adsorption coating with chelating reagents (dithiocarbamates and organic extract) on the flotation properties of mineral aggregates was established.
The results of flotation enrichment of the refractory gold-bearing ore of the Malinovskoe deposit showed (Table 2) that the replacement of part of the butyl xanthogenate with cyanethyldiethyldithiocarbamate led to an increase in the gold content in the concentrate by 0.55 g/t with an increase in extraction by 2.4 %.
Table 2
The results of flotation enrichment of the Malinovskoe deposit ore
|
Reagent mode |
Products |
Output, % |
Au content, g/t |
Au extraction, % |
|
PBX |
Concentrate |
17.04 |
11.57 |
85.30 |
|
Tailings |
82.96 |
0.41 |
14.81 |
|
|
Ore |
100 |
2.31 |
100 |
|
|
CEDEDTC, PBX |
Concentrate |
17.22 |
12.12 |
87.67 |
|
Tailings |
82.78 |
0.35 |
12.33 |
|
|
Ore |
100 |
2.38 |
100 |
|
|
CEDEDTC, HE, PBX |
Concentrate |
17.30 |
12.25 |
91.34 |
|
Tailings |
82.7 |
0.24 |
8.66 |
|
|
Ore |
100 |
2.32 |
100 |
The supply of a plant modifier enhanced the effect of the combination of collectors and increased gold extraction by 6 % to 91.3 %, while the Au content in the tailings decreased from 0.41 to 0.24 g/t compared to the base experiment.
Conclusion
In the rapidly developing world of high-tech manufacturing, the need for strategic metals has increased. The expansion of the range of domestic flotation reagents, which are not inferior to foreign analogues in efficiency, is becoming particularly relevant. The development and scientific justification of new flotation chelating reagents and their combinations is based on fundamental studies of phase and chemical transformations on the surface of minerals in interaction with reagents. Obtaining reliable knowledge is possible only through the use of modern high-resolution physical and chemical analysis methods. Understanding these processes is the key to creating reagents with selective action.
The results of these studies confirmed the individual nature of the fixation of chelating reagents-collectors – derivatives of dithiocarbamates MDTC and CEDEDTC on gold-containing sulfide minerals that are part of the multicomponent ore of the Malinovskoe deposit, with the formation of stable complex compounds that increase the extraction of gold into the flotation concentrate. The replacement of a part of butyl xanthogenate with CEDEDTC led to an increase in the gold content in the concentrate by 0.55 g/t and an increase in extraction by 2.4 %. The plant modifier, adsorbed on gold-containing sulfides, enhances the effect of the combination of collectors. The supply of a plant modifier enhanced the effect of the collector combination and increased gold extraction by 6 % to 91.3 %, while the Au content in the tailings decreased by 1.7 times.
The development of flotation chelating reagents and their compositions is a scientific task of great practical importance for the mining industry. Modern research in this field relies on advanced technologies to create environmentally friendly and highly efficient solutions.
The development of research on new reagent compositions will reduce the loss of strategic metals during the processing of difficult-to-enrich mineral raw materials.
References
- Chanturiya V.A., Kozlov A.P. Current problems and priority areas of scientific research in the field of mineral processing. Materialy Rossiiskogo soveshchaniya s mezhdunarodnym uchastiem “Rol tekhnologicheskoi mineralogii v ratsionalnom nedropolzovanii”. Moskva, 15-16 maya 2018 g. Moscow: VIMS, 2018, p. 11-15 (in Russian).
- Chanturia V.A. Scientific substantiation and development of innovative approaches to integrated mineral processing. Gornyi zhurnal. 2017. N 1, p. 7-13 (in Russian). DOI: 10.17580/gzh.2017.11.01
- Chanturiya V.A. Scientific substantiation and development of innovative processes for the extraction of zirconium and rare earth elements in the deep and comprehensive treatment of eudialyte concentrate. Journal of Mining Institute. 2022. Vol. 256, p. 505-516. DOI:10.31897/PMI.2022.31
- Chanturia V.A., Nikolaev A.I., Aleksandrova T.N. Innovative Environmentally Safe Processes for the Extraction of Rare and Rare-Earth Elements from Complex Ores of Perplexed Material Composition. Geology of Ore Deposits. 2023. Vol. 65. N 5, p. 425-437. DOI: 10.1134/S1075701523050045
- Ivanik S.A., Ilyukhin D.A. Flotation extraction of elemental sulfur from gold-bearing cakes. Journal of Mining Institute. 2020. Vol. 242, p. 202-208. DOI: 10.31897/PMI.2020.2.202
- Chanturiya V.A., Kondratiev S.A. Contemporary Understanding and Developments in the Flotation Theory of Non-Ferrous Ores. Mineral Processing and Extractive Metallurgy Review. 2019. Vol. 40. Iss. 6, p. 390-401. DOI: 10.1080/08827508.2019.1657863
- Bocharov V.A., Ignatkina V.A., Kayumov A.A. Theory and practice of mineral separation of massive refractory polymetallic ores of non-ferrous metals. Moscow: Gornaya kniga, 2019, p. 432 (in Russian).
- Aleksandrova T.N., Prokhorova E.O. Modification of properties of rock-forming minerals during flotation. Mining Informa-tional and Analytical Bulletin. 2023. N 12, p. 123-138 (in Russian). DOI: 10.25018/0236_1493_2023_12_0_123
- Aleksandrova T.N., Orlova A.V., Taranov V.A. Enhancement of copper concentration efficiency in complex ore processing by the reagent regime variation. Journal of Mining Science. 2020. Vol. 56. N 6, p. 982-989. DOI: 10.1134/S1062739120060101
- Solozhenkin P.M. Effects of lead and copper cations on antimonite flotation. Obogashchenie rud. 2024. N 1, p. 39-43 (in Russian). DOI: 10.17580/or.2024.01.07
- Ryaboy V.I., Shepeta E.D. Dialkyldithiophosphates surface activity and hydrophobic properties effects upon copper arsenic-containing ores flotation. Obogashchenie rud. 2016. N 4, p. 29-34 (in Russian). DOI: 10.17580/or.2016.04.05
- Shumilova L.V., Kostikova O.S. Sulfidization of Silver-Polymetallic Ores of “Goltsovoe” Deposit for Decreasing Loss of Silver in Mill Tailings. Journal of Mining Institute. 2018. Vol. 230, p. 160-166. DOI: 10.25515/PMI.2018.2.160
- BalaRamesh P., Venkatesh P., Jabbar A.A. Influence of Dithiocarbamate on Metal Complex and Thin Film Depositions. International Journal of Innovative Research in Science, Engineering and Technology. 2014. Vol. 3. N 8, p. 15301-15309. DOI: 10.15680/IJIRSET.2014.0308033
- Ly N.H., Nguyen T.D., Zoh K.-D., Joo S.-W. Interaction between Diethyldithiocarbamate and Cu(II) on Gold in Non-Cyanide Wastewater. Sensors. 2017. Vol. 17. Iss. 11. N 2628. DOI: 10.3390/s17112628
- Chanturia V.A., Getman V.V. Experimental investigation of interaction between modified thermomorphic polymers, gold and platinum in dressing of rebellious precious metal ore. Journal of Mining Science. 2015. Vol. 51. N 3, p. 580-585. DOI: 10.1134/S1062739115030217
- Wei Sung Ng, Connal L.A., Forbes E., Franks G.V. A review of temperature-responsive polymers as novel reagents for solid-liquid separation and froth flotation of minerals. Minerals Engineering. 2018. Vol. 123, p. 144-159. DOI: 10.1016/j.mineng.2018.03.027
- Semushkina L., Abdykirova G., Mukhanova A., Mukhamedilova A. Improving the Copper-Molybdenum Ores Flotation Technology Using a Combined Collecting Agent. Minerals. 2022. Vol. 12. Iss. 11. N 1416. DOI: 10.3390/min12111416
- Aleksandrova T., Nikolaeva N., Afanasova A. et al. Extraction of Low-Dimensional Structures of Noble and Rare Metals from Carbonaceous Ores Using Low-Temperature and Energy Impacts at Succeeding Stages of Raw Material Transformation. Minerals. 2023. Vol. 13. Iss. 1. N 84. DOI: 10.3390/min13010084
- Spooren J., Binnemans K., Björkmalm J. et al. Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: technology development trends. Resources, Conservation and Recycling. 2020. Vol. 160. N 104919. DOI: 10.1016/j.resconrec.2020.104919
- Milosavljević M.M., Marinković A.D., Rančić M. et al. New Eco-Friendly Xanthate-Based Flotation Agents. Minerals. 2020. Vol. 10. Iss. 4. N 350. DOI: 10.3390/min10040350
- Marfitsin A. The economic effect of using Flotent reagents for the mining industry. Zoloto i tekhnologii. 2020. N 4 (50), p. 102-105 (in Russian).
- Bocharov V.A., Ignatkina V.A., Kayumov A.A Methods of gold recovery during the concentration of refractory gold-bearing pyritic copper-zinc ores. Part 1. Analysis of practice and choice of ways of selective recovery of mineral phases of gold from pyritic copper-zinc ores. Tsvetnye metally. 2017. N 4, p. 11-16. DOI: 10.17580/tsm.2017.04.01
- Zharolla N.D., Yergeshev A.R., Ignatkina V.A. Estimation of selectivity of sulfhydryl collectors on a dithiophosphate basis. Mining Informational and Analytical Bulletin. 2020. N 11, p. 14-26 (in Russian). DOI: 10.25018/0236-1493-2020-11-0-14-26
- Yushina T.I., Purev B., D’Elia Yanes K.S., Malofeeva P.R. Improvement of porphyry copper flotation efficiency with auxiliary collecting agents based on acetylene alcohols. Eurasian Mining. 2019. N 1, p. 25-30. DOI: 10.17580/em.2019.01.06
- Kondratev S.A., Semyanova D.V. Relation between Hydrocarbon Radical Structure and Collecting Abilities of Flotation Agent. Journal of Mining Science. 2018. Vol. 54. N 6, p. 1024-1034. DOI: 10.1134/S1062739118065180
- Kondratev S.A. The physical form of the reagent sorption and its purpose in flotation. Novosibirsk: Nauka, 2018, p. 182 (in Russian).
- Mitrofanova G.V., Chernousenko E.V., Kompanchenko A.A., Kalugin A.I. Specific action of collector from phosphoric acid alkyl esters class in flotation of apatite-nepheline ores. Journal of Mining Institute. 2024. Vol. 268, p. 637-645.
- Burdonov A.E., Vchislo N.V., Verochkina E.A., Rozentsveig I.B. Synthesis of new dithiocarbamate and xanthate complexes and their application in enrichment processes. Proceedings of Universities. Applied Chemistry and Biotechnology. 2023. Vol. 13. N 2, p. 160-171 (in Russian). DOI: 10.21285/2227-2925-2023-13-2-160-171
- Kurkov A.V., Anufrieva S.I., Temnov A.V. Prospects for creation and implementation of integrated technologies for pro-cessing of subsoil waste use. Sustainable Development of Mountain Territories. 2021. Vol. 13. N 2 (48), p. 179-187 (in Russian). DOI: 10.21177/1998-4502-2021-13-2-179-187
- Kurkov A.V., Mamoshin M.Yu., Anufrieva S.I., Avdonin G.I. Molecular recognition ionites – a breakthrough direction for the selective extraction of high-tech metals. Prospect and protection of mineral resources. 2020. N 3, p. 35-46 (in Russian).
- Shangyong Lin, Runqing Liu, Yongjie Bu et al. Oxidative Depression of Arsenopyrite by Using Calcium Hypochlorite and Sodium Humate. Minerals. 2018. Vol. 8. Iss. 10. N 463. DOI: 10.3390/min8100463
- Rui-zeng Liu, Wen-qing Qin, Fen Jiao et al. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate. Transactions of Nonferrous Metals Society of China. 2016. Vol. 26. Iss. 1, p. 265-271. DOI: 10.1016/S1003-6326(16)64113-4
- Lopéz R., Jordão H., Hartmann R. et al. Study of butyl-amine nanocrystal cellulose in the flotation of complex sulphide ores. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2019. Vol. 579. N 123655. DOI: 10.1016/j.colsurfa.2019.123655
- Xiao Jingjing, Liu Guangyi, Zhong Hong et al. The flotation behavior and adsorption mechanism of O-isopropyl-S-[2-(hydroxyimino) propyl] dithiocarbonate ester to chalcopyrite. Journal of the Taiwan Institute of Chemical Engineers. 2017. Vol. 71, p. 38-46. DOI: 10.1016/j.jtice.2016.12.022
- Tijsseling L.T., Dehaine Q., Rollinson G.K., Glass H.J. Flotation of mixed oxide sulphide copper-cobalt minerals using xanthate, dithiophosphate, thiocarbamate and blended collectors. Minerals Engineering. 2019. Vol. 138, p. 246-256. DOI: 10.1016/j.mineng.2019.04.022
- Kyaw Z.Y., Tiagalieva Z.A., Htet Z., Phyo K.K. Improvement of reagent flotation modes of sphalerite and pyrite from deposits of copper-zinc pyrite, polymetallic copper-zinc pyrite and polymetallic ores. IOP Conference Series: Earth and Environmental Science. 2021. Vol. 942. N 012004. DOI: 10.1088/1755-1315/942/1/012004
- Sarquís P.E., Menéndez-Aguado J.M., Mahamud M.M., Dzioba R. Tannins: the organic depressants alternative in selective flotation of sulfides. Journal of Cleaner Production. 2014. Vol. 84, p. 723-726. DOI: 10.1016/j.jclepro.2014.08.025
- Ivanova T.A., Zimbovsky I.G., Koporulina E.V. Enhancing Multipurpose Use of Cow-Parsnip in Processing of Gold-Bearing Sulfides. Journal of Mining Science. 2017. Vol. 53. N 2, p. 327-333. DOI: 10.1134/S1062739117022199
- Matveeva T.N., Chanturiya V.A., Getman V.V. et al. The Effect of Complexing Reagents on Flotation of Sulfide Minerals and Cassiterite from TinSulfide Tailings. Mineral Processing and Extractive Metallurgy Review. 2022. Vol. 43. Iss. 3, p. 346-359. DOI: 10.1080/08827508.2020.1858080
- Matveeva T.N., Gromova N.K., Lantsova L.B., Gladysheva O.I. Mechanism of Interaction between Morpholine Dithiocarbamate and Cyanoethyl Diethyldithiocarbamate Reagents and Low-Dimensional Gold on the Surface of Sulfide Minerals in Flotation of Difficult Gold-Bearing Ore. Journal of Mining Science. 2022. Vol. 58. N 4, p. 610-618. DOI: 10.1134/S106273912204010X
- Matveeva T.N., Gromova N.K., Lantsova L.B. Experimental proof of applicability of cyclic and aliphatic dithiocarbamate collectors in gold-bearing sulphide recovery from complex ore. Journal of Mining Science. 2021. Vol. 57. N 1, p. 123-130. DOI: 10.1134/S1062739121010130
- Matveeva T.N., Gromova N.K., Minaev V.A. Quantitative evaluation of adsorption layer of combined diethyldithiocarbamate on chalkopyrite and arsenopyrite by method of measuring the parameters of surface relief. Tsvetnye metally. 2018. N 7, p. 27-32 (in Russian). DOI: 10.17580/tsm.2018.07.04
- Dobroshevskii K.N. Geological position and mineralogical and geochemical features of the Malinovskoye gold deposit (Central Primorye): Avtoref. dis. … kand. geol.-mineral. nauk. Vladivostok: Dalnevostochnyi geologicheskii institut Dalnevostochnogo otdeleniya RAN, 2019, p. 30 (in Russian).
